COVID-19 Seroprevalence and Active Infection in an Asymptomatic Population
- PMID: 34589507
- PMCID: PMC8473750
- DOI: 10.3389/fmed.2021.749732
COVID-19 Seroprevalence and Active Infection in an Asymptomatic Population
Abstract
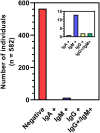
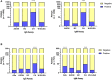
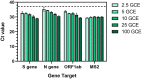
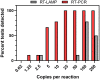
In response to the COVID-19 pandemic, immediate and scalable testing solutions are needed to direct return to full capacity planning in the general public and across the Department of Defense (DoD). To fully understand the extent to which a population has been affected by COVID-19, active monitoring approaches require an estimation of overall seroprevalence in addition to accurate, affordable, and rapid tests to detect current SARS-CoV-2 infection. In this study, researchers in the Air Force Research Laboratory's 711th Human Performance Wing, Airman Systems Directorate evaluated the performance of various testing methods for the detection of SARS-CoV-2 antibodies and viral RNA in asymptomatic adults working at Wright-Patterson Air Force Base and the surrounding area during the period of 23 July 2020-23 Oct 2020. Altogether, there was a seroprevalance of 3.09% and an active infection rate of 0.5% (determined via the testing of saliva samples) amongst individuals tested, both of which were comparable to local and national averages at the time. This work also presents technical and non-technical assessments of various testing strategies as compared to the gold standard approaches (e.g., lateral flow assays vs. ELISA and RT-LAMP vs. RT-PCR) in order to explore orthogonal supply chains and fieldability. Exploration and validation of multiple testing strategies will allow the DoD and other workforces to make informed responses to COVID-19 and future pandemics.
Keywords: COVID-19; SARS-CoV-2; antibodies; asymptomatic; saliva; seroprevalance; testing.
Copyright © 2021 Breedon, Saldanha, Salisbury, Metzger, Werry, McPherson, Irvin, Davis, Bogner, Braddock, Salter, Grigsby, Hart and Pangburn.
Conflict of interest statement
ABre, DM, CM, CD, and ABra were employed by UES, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- US Centers for Disease Control and Prevention . COVID Data Tracker. (2021). Available online at: https://covid.cdc.gov/covid-data-tracker (accessed June 2, 2021).
-
- US Department of Defense . Coronavirus: DOD Response. (2021). Available online at: https://www.defense.gov/Explore/Spotlight/Coronavirus/ (accessed June 2, 2021).
LinkOut - more resources
Full Text Sources
Miscellaneous

