Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier
- PMID: 34589512
- PMCID: PMC8475765
- DOI: 10.3389/fnut.2021.718356
Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier
Erratum in
-
Corrigendum: Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier.Front Nutr. 2021 Nov 1;8:790387. doi: 10.3389/fnut.2021.790387. eCollection 2021. Front Nutr. 2021. PMID: 34790692 Free PMC article.
Abstract
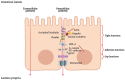
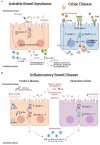
The intestinal epithelial barrier (IEB) is one of the largest interfaces between the environment and the internal milieu of the body. It is essential to limit the passage of harmful antigens and microorganisms and, on the other side, to assure the absorption of nutrients and water. The maintenance of this delicate equilibrium is tightly regulated as it is essential for human homeostasis. Luminal solutes and ions can pass across the IEB via two main routes: the transcellular pathway or the paracellular pathway. Tight junctions (TJs) are a multi-protein complex responsible for the regulation of paracellular permeability. TJs control the passage of antigens through the IEB and have a key role in maintaining barrier integrity. Several factors, including cytokines, gut microbiota, and dietary components are known to regulate intestinal TJs. Gut microbiota participates in several human functions including the modulation of epithelial cells and immune system through the release of several metabolites, such as short-chain fatty acids (SCFAs). Mediators released by immune cells can induce epithelial cell damage and TJs dysfunction. The subsequent disruption of the IEB allows the passage of antigens into the mucosa leading to further inflammation. Growing evidence indicates that dysbiosis, immune activation, and IEB dysfunction have a role in several diseases, including irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and gluten-related conditions. Here we summarize the interplay between the IEB and gut microbiota and mucosal immune system and their involvement in IBS, IBD, and gluten-related disorders.
Keywords: IBD; IBS; celiac disease; gut microbiota; intestinal epithelial barrier; mucosal immune system; non-celiac gluten sensitivity.
Copyright © 2021 Barbara, Barbaro, Fuschi, Palombo, Falangone, Cremon, Marasco and Stanghellini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

