Cell stiffness predicts cancer cell sensitivity to ultrasound as a selective superficial cancer therapy
- PMID: 34589601
- PMCID: PMC8459597
- DOI: 10.1002/btm2.10226
Cell stiffness predicts cancer cell sensitivity to ultrasound as a selective superficial cancer therapy
Abstract
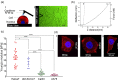
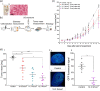
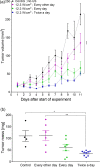
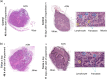
We hypothesize that the biomechanical properties of cells can predict their viability, with Young's modulus representing the former and cell sensitivity to ultrasound representing the latter. Using atomic force microscopy, we show that the Young's modulus stiffness measure is significantly lower for superficial cancer cells (squamous cell carcinomas and melanoma) compared with noncancerous keratinocyte cells. In vitro findings reveal a significant difference between cancerous and noncancerous cell viability at the four ultrasound energy levels evaluated, with different cell lines exhibiting different sensitivities to the same ultrasound intensity. Young's modulus correlates with cell viability (R 2 = 0.93), indicating that this single biomechanical property can predict cell sensitivity to ultrasound treatment. In mice, repeated ultrasound treatment inhibits tumor growth without damaging healthy skin tissue. Histopathological tumor analysis indicates ultrasound-induced focal necrosis at the treatment site. Our findings provide a strong rationale for developing ultrasound as a noninvasive selective treatment for superficial cancers.
Keywords: AFM measurements; mechanical properties of cancer cells; noninvasive therapy; selective cancer therapy; superficial cancer; ultrasound.
© 2021 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
J. K. is an inventor on a U.S. patent application 14/198,701 on low intensity ultrasound therapy of hyperproliferative diseases and disorders. The authors declare no other conflict of interests.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

