E3 ligase activity of Carboxyl terminus of Hsc70 interacting protein (CHIP) in Wharton's jelly derived mesenchymal stem cells improves their persistence under hyperglycemic stress and promotes the prophylactic effects against diabetic cardiac damages
- PMID: 34589606
- PMCID: PMC8459600
- DOI: 10.1002/btm2.10234
E3 ligase activity of Carboxyl terminus of Hsc70 interacting protein (CHIP) in Wharton's jelly derived mesenchymal stem cells improves their persistence under hyperglycemic stress and promotes the prophylactic effects against diabetic cardiac damages
Abstract
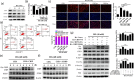
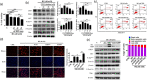
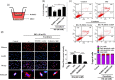
Recent studies indicate that umbilical cord stem cells are cytoprotective against several disorders. One critical limitation in using stem cells is reduction in their viability under stressful conditions, such as diabetes. However, the molecular intricacies responsible for diabetic conditions are not fully elucidated. In this study, we found that high glucose (HG) conditions induced loss of chaperone homeostasis, stabilized PTEN, triggered the downstream signaling cascade, and induced apoptosis and oxidative stress in Wharton's jelly derived mesenchymal stem cells (WJMSCs). Increased Carboxyl terminus of Hsc70 interacting protein (CHIP) expression promoted phosphatase and tensin homolog (PTEN) degradation via the ubiquitin-proteasome system and shortened its half-life during HG stress. Docking studies confirmed the interaction of CHIP with PTEN and FOXO3a with the Bim promoter region. Further, it was found that the chaperone system is involved in CHIP-mediated PTEN proteasomal degradation. CHIP depletion stabilizes PTEN whereas PTEN inhibition showed an inverse effect. CHIP overactivation suppressed the binding of FOXO3a with bim. Coculturing CHIP overexpressed WJMSCs suppressed HG-induced apoptosis and oxidative stress in embryo derived cardiac cell lines. CHIP overexpressing and PTEN silenced WJMSCs ameliorated diabetic effects in streptozotocin (STZ) induced diabetic rats and further improved their body weight and heart weight, and rescued from hyperglycemia-induced cardiac injury. Considering these, the current study suggests that CHIP confers resistance to apoptosis and acts as a potentiation factor in WJMSCs to provide protection from degenerative effects of diabetes.
Keywords: Wharton's jelly derived mesenchymal stem cells; apoptosis; carboxyl terminus of Hsc70 interacting protein; diabetes; phosphatase and tensin homolog.
© 2021 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

