Lipopolysaccharide animal models of Parkinson's disease: Recent progress and relevance to clinical disease
- PMID: 34589845
- PMCID: PMC8474547
- DOI: 10.1016/j.bbih.2020.100060
Lipopolysaccharide animal models of Parkinson's disease: Recent progress and relevance to clinical disease
Abstract
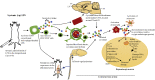
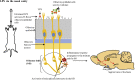
Parkinson's disease (PD) is one of the most common neurodegenerative movement disorders which is characterised neuropathologically by progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the presence of Lewy bodies (made predominately of α-synuclein) in the surviving neurons. Animal models of PD have improved our understanding of the disease and have played a critical role in the development of neuroprotective agents. Neuroinflammation has been strongly implicated in the pathogenesis of PD, and recent studies have used lipopolysaccharide (LPS), a component of gram-negative bacteria and a potent activator of microglia cells, to mimic the inflammatory events in clinical PD. Modulating the inflammatory response could ameliorate PD associated complications and thus, it is essential to understand the extent to which LPS models reflect human PD. This review will outline the routes of administration of LPS such as stereotaxic, systemic and intranasal, their ability to recapitulate neuropathological markers of PD, and mechanisms of LPS induced toxicity. We will also discuss the ability of the models to replicate motor symptoms and non-motor symptoms of PD such as gastrointestinal dysfunction, olfactory dysfunction, anxiety, depression and cognitive dysfunction.
Keywords: Animal models; Lipopolysaccharide; Motor symptoms; Non-motor symptoms; Parkinson’s disease.
© 2020 Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Hernández-Romero M.d.C., Argüelles S., Villarán R.F., De Pablos R.M., Delgado-Cortés M.J., Santiago M., Herrera A.J., Cano J., Machado A. Simvastatin prevents the inflammatory process and the dopaminergic degeneration induced by the intranigral injection of lipopolysaccharide. J. Neurochem. 2008;105:445–459. - PubMed
-
- Badshah H., Ali T., Rehman S.-u., Amin F.-u., Ullah F., Kim T.H., Kim M.O. Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. J. Neuroimmune Pharmacol. 2016;11:48–60. doi: 10.1007/s11481-015-9623-z. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

