Developmental ablation of mature oligodendrocytes exacerbates adult CNS demyelination
- PMID: 34589870
- PMCID: PMC8474627
- DOI: 10.1016/j.bbih.2020.100110
Developmental ablation of mature oligodendrocytes exacerbates adult CNS demyelination
Abstract
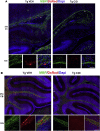
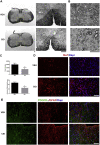
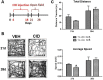
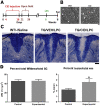
Multiple sclerosis (MS) is a CNS neurodegenerative autoimmune disease characterized by loss of oligodendrocytes and myelin in the brain and the spinal cord that results in localized functional deficits. Several risk factors have been associated with MS, however none fully explain the enhanced susceptibility seen in older individuals. Epidemiological data, based on geographical prevalence studies suggest that susceptibility is established early in life and frequently long before the diagnosis of disease raising the possibility that developmental events influence adult disease onset and progression. Here we test the hypothesis that selective loss of mature oligodendrocytes during postnatal development results in enhanced susceptibility to a demyelinating insult to the mature CNS. A transgenic mouse model was utilized to specifically induce apoptotic cell death in a subset of mature oligodendrocytes (MBP-iCP9) during the first 2 postnatal weeks followed by either a local LPC spinal cord injection or the induction of EAE in the adult animal. Immunostaining, immunoblotting, behavioral testing, and electron microscopy were utilized to examine the differences in the response between animals with developmental loss of oligodendrocytes and controls. We show that during development, oligodendrocyte apoptosis results in transient reductions in myelination and functional deficits that recover after 10-14 days. Compared to animals in which oligodendrocyte development was unperturbed, animals subjected to postnatal oligodendrocyte loss showed delayed recovery from an LPC lesion to the mature spinal cord. Unexpectedly, the induction and severity of MOG induced EAE was not significantly altered in animals following oligodendrocyte developmental loss even though there was a substantial increase in spinal cord tissue damage and CNS inflammation. It is unclear why the elevated glial responses seen in developmentally compromised animals were not reflected in enhanced functional deficits. These observations suggest that developmental loss of oligodendrocytes results in long lasting tissue changes that alter its response to subsequent insults and the capacity for repair in the adult.
Keywords: EAE; Inflammation; LPC; Multiple sclerosis; Priming.
© 2020 The Authors.
Conflict of interest statement
We do not have any conflict of interest regarding this study.
Figures




Similar articles
-
Apoptosis of Oligodendrocytes during Early Development Delays Myelination and Impairs Subsequent Responses to Demyelination.J Neurosci. 2015 Oct 14;35(41):14031-41. doi: 10.1523/JNEUROSCI.1706-15.2015. J Neurosci. 2015. PMID: 26468203 Free PMC article.
-
The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review.
-
Oligodendrocyte-specific ATF4 inactivation does not influence the development of EAE.J Neuroinflammation. 2019 Feb 1;16(1):23. doi: 10.1186/s12974-019-1415-6. J Neuroinflammation. 2019. PMID: 30709400 Free PMC article.
-
Activating transcription factor 6α deficiency exacerbates oligodendrocyte death and myelin damage in immune-mediated demyelinating diseases.Glia. 2018 Jul;66(7):1331-1345. doi: 10.1002/glia.23307. Epub 2018 Feb 13. Glia. 2018. PMID: 29436030 Free PMC article.
-
Contribution of Spinal Cord Oligodendrocytes to Neuroinflammatory Diseases and Pain.Curr Med Chem. 2019;26(31):5781-5810. doi: 10.2174/0929867325666180522112441. Curr Med Chem. 2019. PMID: 29788868 Review.
Cited by
-
Role of Nuclear Receptors on the Progression of Multiple Sclerosis: A Review.Cell Mol Neurobiol. 2025 Jun 17;45(1):58. doi: 10.1007/s10571-025-01563-z. Cell Mol Neurobiol. 2025. PMID: 40526306 Free PMC article. Review.
-
Brain development and bioenergetic changes.Neurobiol Dis. 2024 Sep;199:106550. doi: 10.1016/j.nbd.2024.106550. Epub 2024 Jun 6. Neurobiol Dis. 2024. PMID: 38849103 Free PMC article. Review.
-
Delimiting MOGAD as a disease entity using translational imaging.Front Neurol. 2023 Dec 7;14:1216477. doi: 10.3389/fneur.2023.1216477. eCollection 2023. Front Neurol. 2023. PMID: 38333186 Free PMC article. Review.
References
-
- Bilbo S.D. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav. Neurosci. 2005;119(1):293–301. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

