Sensory filtering disruption caused by poly I:C - Timing of exposure and other experimental considerations
- PMID: 34589898
- PMCID: PMC8474281
- DOI: 10.1016/j.bbih.2020.100156
Sensory filtering disruption caused by poly I:C - Timing of exposure and other experimental considerations
Abstract
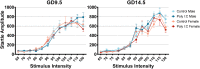
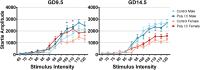
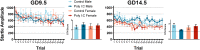
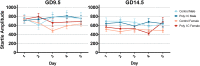
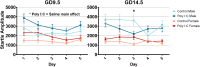
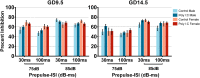
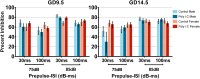
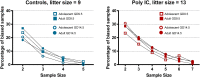
Maternal immune activation (MIA) in response to infection during pregnancy has been linked through various epidemiological and preclinical studies to an increased risk of neurodevelopmental disorders such as autism spectrum disorder (ASD) and schizophrenia in exposed offspring. Sensory filtering disruptions occur in both of these disorders and are typically measured using the acoustic startle response in both humans and rodents. Our study focuses on characterizing the baseline reactivity, habituation and prepulse inhibition (PPI) of the acoustic startle response following exposure to MIA. We induced MIA using polyinosinic: polycytidylic acid (poly I:C) at gestational day (GD) 9.5 or 14.5, and we tested sensory filtering phenotypes in adolescent and adult offspring. Our results show that startle reactivity was robustly increased in adult GD9.5 but not GD14.5 poly I:C offspring. In contrast to some previous studies, we found no consistent changes in short-term habituation, long-term habituation or prepulse inhibition of startle. Our study highlights the importance of MIA exposure timing and discusses sensory filtering phenotypes as they relate to ASD, schizophrenia and the poly I:C MIA model. Moreover, we analyze and discuss the potential impact of between- and within-litter variability on behavioural findings in poly I:C studies.
Keywords: Autism Spectrum Disorder; Habituation; Litter variability; Neurodevelopmental disorder; Poly I:C; Prepulse inhibition; Schizophrenia; Sensorimotor gating; Startle.
© 2020 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Investigating behavioral phenotypes related to autism spectrum disorder in a gene-environment interaction model of Cntnap2 deficiency and Poly I:C maternal immune activation.Front Neurosci. 2023 Mar 14;17:1160243. doi: 10.3389/fnins.2023.1160243. eCollection 2023. Front Neurosci. 2023. PMID: 36998729 Free PMC article.
-
Interleukin 15 modulates the effects of poly I:C maternal immune activation on offspring behaviour.Brain Behav Immun Health. 2022 May 18;23:100473. doi: 10.1016/j.bbih.2022.100473. eCollection 2022 Aug. Brain Behav Immun Health. 2022. PMID: 35668725 Free PMC article.
-
Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring.Behav Brain Res. 2008 Jun 26;190(1):156-9. doi: 10.1016/j.bbr.2008.02.021. Epub 2008 Feb 20. Behav Brain Res. 2008. PMID: 18367260
-
Maternal Immune Activation by Poly I:C as a preclinical Model for Neurodevelopmental Disorders: A focus on Autism and Schizophrenia.Neurosci Biobehav Rev. 2020 Jun;113:546-567. doi: 10.1016/j.neubiorev.2020.04.012. Epub 2020 Apr 19. Neurosci Biobehav Rev. 2020. PMID: 32320814 Review.
-
Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies.Psychopharmacology (Berl). 2001 Jul;156(2-3):234-58. doi: 10.1007/s002130100810. Psychopharmacology (Berl). 2001. PMID: 11549226 Review.
Cited by
-
Characterizing maternal isolation-induced ultrasonic vocalizations in a gene-environment interaction rat model for autism.Genes Brain Behav. 2023 Jun;22(3):e12841. doi: 10.1111/gbb.12841. Epub 2023 Feb 7. Genes Brain Behav. 2023. PMID: 36751016 Free PMC article.
-
Investigating behavioral phenotypes related to autism spectrum disorder in a gene-environment interaction model of Cntnap2 deficiency and Poly I:C maternal immune activation.Front Neurosci. 2023 Mar 14;17:1160243. doi: 10.3389/fnins.2023.1160243. eCollection 2023. Front Neurosci. 2023. PMID: 36998729 Free PMC article.
-
Interleukin 15 modulates the effects of poly I:C maternal immune activation on offspring behaviour.Brain Behav Immun Health. 2022 May 18;23:100473. doi: 10.1016/j.bbih.2022.100473. eCollection 2022 Aug. Brain Behav Immun Health. 2022. PMID: 35668725 Free PMC article.
-
Neurodevelopmental signatures of narcotic and neuropsychiatric risk factors in 3D human-derived forebrain organoids.Mol Psychiatry. 2021 Dec;26(12):7760-7783. doi: 10.1038/s41380-021-01189-9. Epub 2021 Jun 22. Mol Psychiatry. 2021. PMID: 34158620 Free PMC article.
-
Establishment of a two-hit mouse model of environmental factor induced autism spectrum disorder.Heliyon. 2024 May 6;10(9):e30617. doi: 10.1016/j.heliyon.2024.e30617. eCollection 2024 May 15. Heliyon. 2024. PMID: 38774072 Free PMC article.
References
-
- Abazyan B., Nomura J., Kannan G., Ishizuka K., Tamashiro K.L., Nucifora F., Pogorelov V., Ladenheim B., Yang C., Krasnova I.N., Cadet J.L., Pardo C., Mori S., Kamiya A., Vogel M.W., Sawa A., Ross C.A., Pletnikov M.V. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol. Psychiatr. 2010;68:1172–1181. doi: 10.1016/j.biopsych.2010.09.022. - DOI - PMC - PubMed
-
- Ballendine S.A., Greba Q., Dawicki W., Zhang X., Gordon J.R., Howland J.G. Behavioral alterations in rat offspring following maternal immune activation and ELR-CXC chemokine receptor antagonism during pregnancy: implications for neurodevelopmental psychiatric disorders. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2015;57:155–165. doi: 10.1016/j.pnpbp.2014.11.002. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

