The Long Half-Life of Programmed Cell Death Protein 1 Inhibitors May Increase the Frequency of Immune-Related Adverse Events After Subsequent EGFR Tyrosine Kinase Inhibitor Therapy
- PMID: 34589912
- PMCID: PMC8474461
- DOI: 10.1016/j.jtocrr.2020.100008
The Long Half-Life of Programmed Cell Death Protein 1 Inhibitors May Increase the Frequency of Immune-Related Adverse Events After Subsequent EGFR Tyrosine Kinase Inhibitor Therapy
Abstract
Introduction: EGFR tyrosine kinase inhibitors are one of the key drugs for treatment of NSCLC with EGFR mutations. In recent times, immune check-point inhibitors (ICIs) have also been widely used for patients with NSCLC. Although a subset of patients obtain benefit from ICIs, adverse events (AEs) that are different from those of cytotoxic chemotherapies may occur. Moreover, some patients develop AEs, which seem to be caused by the previously discontinued nivolumab.
Methods: We identified patients with NSCLC who developed AEs, which started shortly after discontinuation of nivolumab and during treatment with osimertinib. We conducted liquid chromatography-mass spectrometry analyses to estimate the concentration of serum nivolumab.
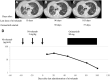
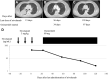
Results: Three patients with AEs were identified. Two patients developed interstitial lung disease (cases 1 and 2) and one developed hepatotoxicity (case 3) during osimertinib therapy initiated after nivolumab administration. They received several treatments, including cytotoxic chemotherapies or EGFR tyrosine kinase inhibitors other than osimertinib, followed by nivolumab for three to five cycles; nevertheless, the disease progressed. After discontinuation of nivolumab, osimertinib was administered from day 22 to 46; but treatment-related toxicities developed 56 to 96 days later. Liquid chromatography-mass spectrometry analyses revealed that the remaining levels of nivolumab in the blood (2.1 μg/mL, 12.8 μg/mL, and 31.1 μg/mL, respectively, for cases 1, 2, and 3) were enough to induce an immune response.
Conclusion: The presence of the ICI antibody that persists even after drug discontinuation may account not only for the prolonged efficacy of these agents but also for the late onset of AEs, especially when the antibodies may have interacted during subsequent treatments.
Keywords: EGFR; Immune-related adverse events; Non−small cell lung cancer; Osimertinib; programmed cell death protein 1.
© 2020 The Authors.
Figures

Similar articles
-
Osimertinib-induced interstitial lung disease in a patient with non-small cell lung cancer pretreated with nivolumab: A case report.Mol Clin Oncol. 2017 Sep;7(3):383-385. doi: 10.3892/mco.2017.1349. Epub 2017 Jul 25. Mol Clin Oncol. 2017. PMID: 28808573 Free PMC article.
-
Case Report: Stevens-Johnson Syndrome and Hepatotoxicity Induced by Osimertinib Sequential to Pembrolizumab in a Patient With EGFR-Mutated Lung Adenocarcinoma.Front Pharmacol. 2021 Aug 12;12:672233. doi: 10.3389/fphar.2021.672233. eCollection 2021. Front Pharmacol. 2021. PMID: 34456717 Free PMC article.
-
The Differences in the Safety and Tolerability of Immune Checkpoint Inhibitors as Treatment for Non-Small Cell Lung Cancer and Melanoma: Network Meta-Analysis and Systematic Review.Front Pharmacol. 2019 Oct 24;10:1260. doi: 10.3389/fphar.2019.01260. eCollection 2019. Front Pharmacol. 2019. PMID: 31708783 Free PMC article.
-
Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer.J Immunother Cancer. 2017 May 16;5:40. doi: 10.1186/s40425-017-0245-2. eCollection 2017. J Immunother Cancer. 2017. PMID: 28515940 Free PMC article.
-
Delayed immune thrombocytopenia after discontinuation of nivolumab therapy: A case report and literature review.J Oncol Pharm Pract. 2021 Sep;27(6):1548-1552. doi: 10.1177/1078155220981155. Epub 2021 Jan 12. J Oncol Pharm Pract. 2021. PMID: 33435825 Review.
Cited by
-
Sequential Treatment with an Immune Checkpoint Inhibitor Followed by a Small-Molecule Targeted Agent Increases Drug-Induced Pneumonitis.Cancer Res Treat. 2021 Jan;53(1):77-86. doi: 10.4143/crt.2020.543. Epub 2020 Aug 6. Cancer Res Treat. 2021. PMID: 32777877 Free PMC article.
-
The toxicity associated with combining immune check point inhibitors with tyrosine kinase inhibitors in patients with non-small cell lung cancer.Front Oncol. 2023 Apr 14;13:1158417. doi: 10.3389/fonc.2023.1158417. eCollection 2023. Front Oncol. 2023. PMID: 37124513 Free PMC article. Review.
-
Treatment-Related Adverse Events of Combination EGFR Tyrosine Kinase Inhibitor and Immune Checkpoint Inhibitor in EGFR-Mutant Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis.Cancers (Basel). 2022 Apr 26;14(9):2157. doi: 10.3390/cancers14092157. Cancers (Basel). 2022. PMID: 35565285 Free PMC article. Review.
-
Pursuit or discontinuation of anti-PD1 after 2 years of treatment in long-term responder patients with non-small cell lung cancer.Ther Adv Med Oncol. 2023 Sep 13;15:17588359231195600. doi: 10.1177/17588359231195600. eCollection 2023. Ther Adv Med Oncol. 2023. PMID: 37720494 Free PMC article.
-
Immune checkpoint inhibition mediated with liposomal nanomedicine for cancer therapy.Mil Med Res. 2023 Apr 28;10(1):20. doi: 10.1186/s40779-023-00455-x. Mil Med Res. 2023. PMID: 37106400 Free PMC article. Review.
References
-
- Takagi T., Yoshida K., Kobayashi H. Durable response after discontinuation of nivolumab therapy in patients with metastatic renal cell carcinoma. Jpn J Clin Oncol. 2018;48:860–863. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

