The Impact of the Availability of Immunotherapy on Patterns of Care in Stage III NSCLC: A Dutch Multicenter Analysis
- PMID: 34590040
- PMCID: PMC8474425
- DOI: 10.1016/j.jtocrr.2021.100195
The Impact of the Availability of Immunotherapy on Patterns of Care in Stage III NSCLC: A Dutch Multicenter Analysis
Abstract
Introduction: Treatment patterns in stage III NSCLC can vary considerably between countries. The PACIFIC trial reported improvements in progression-free and overall survival with adjuvant durvalumab after concurrent chemoradiotherapy (CCRT). We studied treatment decision-making by three Dutch regional thoracic multidisciplinary tumor boards between 2015 and 2019, to identify changes in practice when adjuvant durvalumab became available.
Methods: Details of patients presenting with stage III NSCLC were retrospectively collected. Both CCRT and multimodality schemes incorporating planned surgery were defined as being radical-intent treatment (RIT).
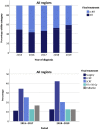
Results: Of 855 eligible patients, most (95%) were discussed at a thoracic multidisciplinary tumor board, which recommended a RIT in 63% (n = 510). Only 52% (n = 424) of the patients finally received a RIT. Predictors for not recommending RIT were age greater than or equal to 70 years, WHO performance score greater than or equal to 2, Charlson comorbidity index greater than or equal to 2 (excluding age), forced expiratory volume in 1 second less than 80% of predicted value, N3 disease, and period of diagnosis. Between 2015 to 2017 and 2018 to 2019, the proportion of patients undergoing CCRT increased from 34% to 42% (p = 0.02) and use of sequential chemoradiotherapy declined (21%-16%, p = 0.05). Rates of early toxicity and 1-year mortality were comparable for both periods. After 2018, 57% of the patients who underwent CCRT (90 of 159) received adjuvant durvalumab.
Conclusions: After publication of the PACIFIC trial, a significant increase was observed in the use of CCRT for patients with stage III NSCLC with rates of early toxicity and mortality being unchanged. Since 2018, 57% of the patients undergoing CCRT went on to receive adjuvant durvalumab. Nevertheless, approximately half of the patients were still considered unfit for a RIT.
Keywords: Immunotherapy; Multidisciplinary tumor board (MDT); Non–small cell lung cancer (NSCLC); Patterns of care; Stage III.
© 2021 The Authors.
Figures


References
-
- Postmus P.E., Kerr K.M., Oudkerk M. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv1–iv21. - PubMed
-
- Antonia S.J., Villegas A., Daniel D. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. - PubMed
-
- Antonia S.J., Villegas A., Daniel D. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. - PubMed
-
- Park K., Vansteenkiste J., Lee K.H. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with locally-advanced unresectable non-small-cell lung cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol. 2020;31:191–201. - PubMed
-
- West H.J. Jarring discordance between idealized and real-world management in stage III non-small-cell lung cancer. JCO Oncol Pract. 2020;16:628–630. - PubMed
LinkOut - more resources
Full Text Sources

