Diversity in Alzheimer's disease drug trials: The importance of eligibility criteria
- PMID: 34590409
- PMCID: PMC8964823
- DOI: 10.1002/alz.12433
Diversity in Alzheimer's disease drug trials: The importance of eligibility criteria
Abstract
Introduction: To generalize safety and efficacy findings, it is essential that diverse populations are well represented in Alzheimer's disease (AD) drug trials. In this review, we aimed to investigate participant diversity in disease-modifying AD trials over time, and the frequencies of participant eligibility criteria.
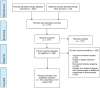
Methods: A systematic review was performed using Medline, Embase, the Cochrane Library, and Clinicaltrials.gov, identifying 2247 records.
Results: In the 101 included AD trials, participants were predominantly White (median percentage: 94.7%, interquartile range: 81.0-96.7%); and this percentage showed no significant increase or decrease over time (2001-2019). Eligibility criteria such as exclusion of persons with psychiatric illness (78.2%), cardiovascular disease (71.3%) and cerebrovascular disease (68.3%), obligated caregiver attendance (80.2%), and specific Mini-Mental State Examination scores (90.1%; no significant increase/decrease over time) may have led to a disproportionate exclusion of ethnoracially diverse individuals.
Discussion: Ethnoracially diverse participants continue to be underrepresented in AD clinical trials. Several recommendations are provided to broaden eligibility criteria.
Keywords: clinical trial; clinical trial protocols; cultural diversity; ethnic groups; phase II; phase III; randomized controlled trials.
© 2021 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
RLvB‐V, JES, ELA, GMB, JMP, EvdB have nothing to disclose (aside from the funding reported in the “Funding Information” section). SF received support to attend meetings/conferences from Alzheimer Nederland and the Erasmus Trustfonds. She served as the executive committee member of the Cultural Diversity & Psychology commission of the Dutch Association of Psychologists and serves as executive committee member to the Diversity and Disparities PIA of ISTAART. NDP is consultant to Boehringer Ingelheim, Amylyx, and Aribio (payments made to his institution). He is co‐PI of studies with EIP Pharma and Fuji Film Toyama Chemical. He serves on the DSMB of Abbvie's M15‐566 trial (payment to his institution). He is CEO and co‐owner of the Brain Research Center, the Netherlands. He is also on the scientific program committee of the Alzheimer's Association. LSS reports grants/contracts by Eisai, Eli Lilly, Roche/Genentech, Biogen, Biohaven, Novartis, and Washington University/NIA‐DIAN‐TU paid to the institution. In addition, he reports consulting fees from Abbott, AC Immune, Avraham Ltd, Boehringer Ingelheim, Cognition Therapeutics, Cortexyme, Eisai, FujiFilm, Immunobrain Checkpoint Ltd, Neurally Inc, Neurim Ltd, Neuronix Ltd, Samus, Takeda, vTv. MRM is the past president of the Hispanic Neuropsychological Society and standing member of the NIH NIA‐T Study Section. MRM also has a relationship with the Alzheimer's Association Harem Community and Academic partnership. MRM received support from NIH/NIA to attend meetings. MRM received payment/honoraria for being panelist/chair/speaker/plenary speaker at the following events: AAIC Neuroscience Next 2020; mid‐year conference of the International Neuropsychological Society 2019; Latinos and Alzheimer's Disease Symposium 2019; International Association of Forensic Mental Health Services Conference 2017; International Neuropsychological Society Annual Conference 2012; International Neuropsychological Society Annual Conference, 2012; 37th Annual Conference of the International Neuropsychological Society Annual Conference, 2010 American Academy of Clinical Neuropsychology annual meeting [2021 delayed due to COVID]; Harvard MGH Psychology Assessment Center Seminar 2021; University of Washington Department of Neurology Grand Rounds [delayed due to COVID 2020]; Annual Conference of the Pacific Northwest Neuropsychological Society, 2020; Annual Conference of the Council of University Directors of Clinical Psychology, 2020; National Academy of Sciences/Simons Foundation: The Science & Entertainment Exchange, 2019; Emory University HIV & Aging Conference; Brown University Alpert Medical School, Department of Psychiatry and Human Behavior Grand Rounds, 2019; Wisconsin Alzheimer's Institute/University of Wisconsin School of Medicine & Public Health 16th Annual Alzheimer's Disease Update Conference, 2018; 38th annual meeting of the National Academy of Neuropsychology, 2018; Council of Science Editors, Technica Editorial Services Webinar. The Peer Review Ecosystem: Where Does Diversity & Inclusion Fit In? 2018; 12) Colloquium Presentation, Dept. of Psychology, Ohio University, Athens, OH, 2018.
Figures
References
-
- Alzheimer's Association . 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:324‐384. - PubMed
-
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63‐75 e2. - PubMed
-
- Canevelli M, Bruno G, Grande G, et al. Race reporting and disparities in clinical trials on Alzheimer's disease: A systematic review. Neurosci Biobehav Rev. 2019;101:122‐128. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical