Molecular photoswitches in aqueous environments
- PMID: 34590636
- PMCID: PMC8591629
- DOI: 10.1039/d0cs00547a
Molecular photoswitches in aqueous environments
Abstract
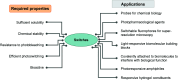
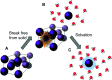
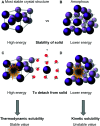
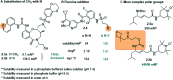
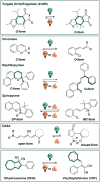
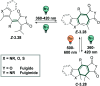
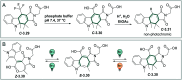
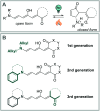
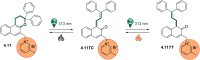
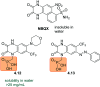
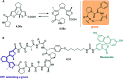
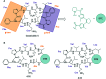
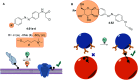
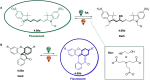
Molecular photoswitches enable dynamic control of processes with high spatiotemporal precision, using light as external stimulus, and hence are ideal tools for different research areas spanning from chemical biology to smart materials. Photoswitches are typically organic molecules that feature extended aromatic systems to make them responsive to (visible) light. However, this renders them inherently lipophilic, while water-solubility is of crucial importance to apply photoswitchable organic molecules in biological systems, like in the rapidly emerging field of photopharmacology. Several strategies for solubilizing organic molecules in water are known, but there are not yet clear rules for applying them to photoswitchable molecules. Importantly, rendering photoswitches water-soluble has a serious impact on both their photophysical and biological properties, which must be taken into consideration when designing new systems. Altogether, these aspects pose considerable challenges for successfully applying molecular photoswitches in aqueous systems, and in particular in biologically relevant media. In this review, we focus on fully water-soluble photoswitches, such as those used in biological environments, in both in vitro and in vivo studies. We discuss the design principles and prospects for water-soluble photoswitches to inspire and enable their future applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



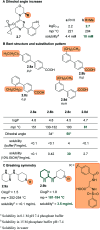



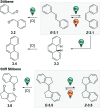


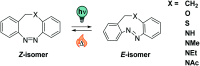

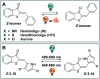









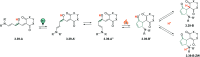

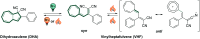




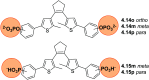

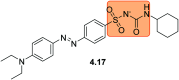

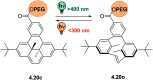
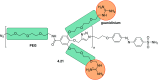
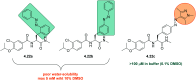
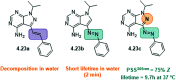

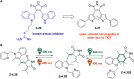




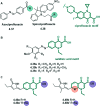

















References
-
- Reichardt K. and Timm L. C., Soil, Plant and Atmosphere, Springer International Publishing, Cham, 2020, pp. 7–13
-
- Molecular Switches, ed. B. L. Feringa and W. R. Browne, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011
-
- Suppan P., Chemistry and Light, Royal Society of Chemistry, Cambridge, 2007
Publication types
LinkOut - more resources
Full Text Sources

