Dye-sensitized solar cells strike back
- PMID: 34590638
- PMCID: PMC8591630
- DOI: 10.1039/d0cs01336f
Dye-sensitized solar cells strike back
Abstract
Dye-sensitized solar cells (DSCs) are celebrating their 30th birthday and they are attracting a wealth of research efforts aimed at unleashing their full potential. In recent years, DSCs and dye-sensitized photoelectrochemical cells (DSPECs) have experienced a renaissance as the best technology for several niche applications that take advantage of DSCs' unique combination of properties: at low cost, they are composed of non-toxic materials, are colorful, transparent, and very efficient in low light conditions. This review summarizes the advancements in the field over the last decade, encompassing all aspects of the DSC technology: theoretical studies, characterization techniques, materials, applications as solar cells and as drivers for the synthesis of solar fuels, and commercialization efforts from various companies.
Conflict of interest statement
GB, HP and AH are co-founders and co-owners of Dyenamo AB.
Figures


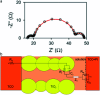
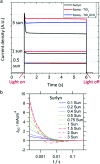
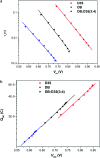

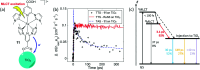






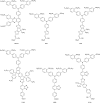


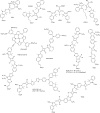
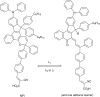
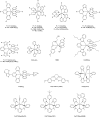

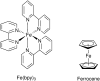

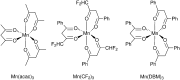
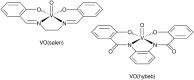









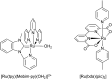

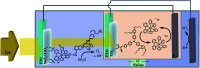






References
-
- Ritchie H., Energy Mix, https://ourworldindata.org/energy-mix
-
- Archer D., Global Warming: Understanding the Forecast, John Wiley & Sons, 2nd edn, 2012
-
- Ritchie H. and Roser M., Renewable Energy, https://ourworldindata.org/renewable-energy
-
- IEA, Renewables 2020, International Energy Agency, 202010.1787/c74616c1-en - DOI
-
- O'Regan B. Grätzel M. Nature. 1991;353:737–740. doi: 10.1038/353737a0. - DOI
Publication types
LinkOut - more resources
Full Text Sources

