Remote Patient Monitoring and Incentives to Support Smoking Cessation Among Pregnant and Postpartum Medicaid Members: Three Randomized Controlled Pilot Studies
- PMID: 34591023
- PMCID: PMC8517817
- DOI: 10.2196/27801
Remote Patient Monitoring and Incentives to Support Smoking Cessation Among Pregnant and Postpartum Medicaid Members: Three Randomized Controlled Pilot Studies
Abstract
Background: Smoking rates among low-income individuals, including those eligible for Medicaid, have not shown the same decrease that is observed among high-income individuals. The rate of smoking among pregnant women enrolled in Medicaid is almost twice that among privately insured women, which leads to significant disparities in birth outcomes and a disproportionate cost burden placed on Medicaid. Several states have identified maternal smoking as a key target for improving birth outcomes and reducing health care expenditures; however, efficacious, cost-effective, and feasible cessation programs have been elusive.
Objective: This study aims to examine the feasibility, acceptability, and effectiveness of a smartwatch-enabled, incentive-based smoking cessation program for Medicaid-eligible pregnant smokers.
Methods: Pilot 1 included a randomized pilot study of smartwatch-enabled remote monitoring versus no remote monitoring for 12 weeks. Those in the intervention group also received the SmokeBeat program. Pilot 2 included a randomized pilot study of pay-to-wear versus pay-to-quit for 4 weeks. Those in a pay-to-wear program could earn daily incentives for wearing the smartwatch, whereas those in pay-to-quit program could earn daily incentives if they wore the smartwatch and abstained from smoking. Pilot 3, similar to pilot 2, had higher incentives and a duration of 3 weeks.
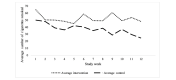
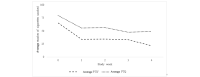
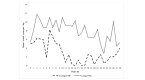
Results: For pilot 1 (N=27), self-reported cigarettes per week among the intervention group declined by 15.1 (SD 27) cigarettes over the study; a similar reduction was observed in the control group with a decrease of 17.2 (SD 19) cigarettes. For pilot 2 (N=8), self-reported cigarettes per week among the pay-to-wear group decreased by 43 cigarettes (SD 12.6); a similar reduction was seen in the pay-to-quit group, with an average of 31 (SD 45.6) fewer cigarettes smoked per week. For pilot 3 (N=4), one participant in the pay-to-quit group abstained from smoking for the full study duration and received full incentives.
Conclusions: Decreases in smoking were observed in both the control and intervention groups during all pilots. The use of the SmokeBeat program did not significantly improve cessation. The SmokeBeat program, remote cotinine testing, and remote delivery of financial incentives were considered feasible and acceptable. Implementation challenges remain for providing evidence-based cessation incentives to low-income pregnant smokers. The feasibility and acceptability of the SmokeBeat program were moderately high. Moreover, the feasibility and acceptability of remote cotinine testing and the remotely delivered contingent financial incentives were successful.
Trial registration: ClinicalTrials.gov NCT03209557; https://clinicaltrials.gov/ct2/show/NCT03209557.
Keywords: financial incentives; incentives; mHealth; maternal smoking; mobile health; mobile phone; postpartum; pregnant; smart devices; smoking; smoking cessation.
©Caroline M Joyce, Kathryn Saulsgiver, Salini Mohanty, Chethan Bachireddy, Carin Molfetta, Mary Steffy, Alice Yoder, Alison M Buttenheim. Originally published in JMIR Formative Research (https://formative.jmir.org), 30.09.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Garrett BE, Dube SR, Babb S, McAfee T. Addressing the social determinants of health to reduce tobacco-related disparities. Nicotine Tob Res. 2015 Aug;17(8):892–7. doi: 10.1093/ntr/ntu266. http://europepmc.org/abstract/MED/25516538 ntu266 - DOI - PMC - PubMed
-
- Kornhauser M, Schneiderman R. How plans can improve outcomes and cut costs for preterm infant care. Manag Care. 2010 Jan;19(1):28–30. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

