AZD1222/ChAdOx1 nCoV-19 vaccination induces a polyfunctional spike protein-specific TH1 response with a diverse TCR repertoire
- PMID: 34591596
- PMCID: PMC9924073
- DOI: 10.1126/scitranslmed.abj7211
AZD1222/ChAdOx1 nCoV-19 vaccination induces a polyfunctional spike protein-specific TH1 response with a diverse TCR repertoire
Abstract
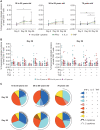
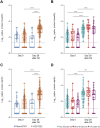
AZD1222 (ChAdOx1 nCoV-19), a replication-deficient simian adenovirus–vectored vaccine, has demonstrated safety, efficacy, and immunogenicity against coronavirus disease 2019 in clinical trials and real-world studies. We characterized CD4+ and CD8+ T cell responses induced by AZD1222 vaccination in peripheral blood mononuclear cells from 296 unique vaccine recipients aged 18 to 85 years who enrolled in the phase 2/3 COV002 trial. Total spike protein–specific CD4+ T cell helper type 1 (TH1) and CD8+ T cell responses were increased in AZD1222-vaccinated adults of all ages after two doses of AZD1222. CD4+ TH2 responses after AZD1222 vaccination were not detected. Furthermore, AZD1222-specific TH1 and CD8+ T cells both displayed a high degree of polyfunctionality in all adult age groups. T cell receptor β (TCRβ) sequences from vaccinated participants mapped against TCR sequences known to react to SARS-CoV-2 revealed substantial breadth and depth across the SARS-CoV-2 spike protein for both AZD1222-induced CD4+ and CD8+ T cell responses. Overall, AZD1222 vaccination induced a polyfunctional TH1-dominated T cell response, with broad CD4+ and CD8+ T cell coverage across the SARS-CoV-2 spike protein.
Figures



Update of
-
T-cell mediated immunity after AZD1222 vaccination: A polyfunctional spike-specific Th1 response with a diverse TCR repertoire.medRxiv [Preprint]. 2021 Jul 13:2021.06.17.21259027. doi: 10.1101/2021.06.17.21259027. medRxiv. 2021. Update in: Sci Transl Med. 2021 Nov 17;13(620):eabj7211. doi: 10.1126/scitranslmed.abj7211. PMID: 34189538 Free PMC article. Updated. Preprint.
References
-
- Dan J. M., Mateus J., Kato Y., Hastie K. M., Yu E. D., Faliti C. E., Grifoni A., Ramirez S. I., Haupt S., Frazier A., Nakao C., Rayaprolu V., Rawlings S. A., Peters B., Krammer F., Simon V., Saphire E. O., Smith D. M., Weiskopf D., Sette A., Crotty S., Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 371, eabf4063 (2021). - PMC - PubMed
-
- Rydyznski Moderbacher C., Ramirez S. I., Dan J. M., Grifoni A., Hastie K. M., Weiskopf D., Belanger S., Abbott R. K., Kim C., Choi J., Kato Y., Crotty E. G., Kim C., Rawlings S. A., Mateus J., Tse L. P. V., Frazier A., Baric R., Peters B., Greenbaum J., Ollmann Saphire E., Smith D. M., Sette A., Crotty S., Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 183, 996–1012.e19 (2020). - PMC - PubMed
-
- Zhou R., To K. K.-W., Wong Y.-C., Liu L., Zhou B., Li X., Huang H., Mo Y., Luk T.-Y., Lau T. T.-K., Yeung P., Chan W.-M., Wu A. K.-L., Lung K.-C., Tsang O. T.-Y., Leung W.-S., Hung I. F.-N., Yuen K.-Y., Chen Z., Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity 53, 864–877.e5 (2020). - PMC - PubMed
-
- Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z., Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 26, 842–844 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

