Immune signatures underlying post-acute COVID-19 lung sequelae
- PMID: 34591653
- PMCID: PMC8763087
- DOI: 10.1126/sciimmunol.abk1741
Immune signatures underlying post-acute COVID-19 lung sequelae
Abstract
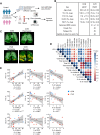
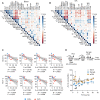
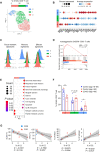
Severe coronavirus disease 2019 (COVID-19) pneumonia survivors often exhibit long-term pulmonary sequelae, but the underlying mechanisms or associated local and systemic immune correlates are not known. Here, we have performed high-dimensional characterization of the pathophysiological and immune traits of aged COVID-19 convalescents, and correlated the local and systemic immune profiles with pulmonary function and lung imaging. We found that chronic lung impairment was accompanied by persistent respiratory immune alterations. We showed that functional severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific memory T and B cells were enriched at the site of infection compared with those of blood. Detailed evaluation of the lung immune compartment revealed that dysregulated respiratory CD8+ T cell responses were associated with the impaired lung function after acute COVID-19. Single-cell transcriptomic analysis identified the potential pathogenic subsets of respiratory CD8+ T cells contributing to persistent tissue conditions after COVID-19. Our results have revealed pathophysiological and immune traits that may support the development of lung sequelae after SARS-CoV-2 pneumonia in older individuals, with implications for the treatment of chronic COVID-19 symptoms.
Figures

References
-
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y., Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 8, –475, 481 (2020). - PMC - PubMed
-
- Wu X., Liu X., Zhou Y., Yu H., Li R., Zhan Q., Ni F., Fang S., Lu Y., Ding X., Liu H., Ewing R. M., Jones M. G., Hu Y., Nie H., Wang Y., 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 9, 747–754 (2021). - PMC - PubMed
-
- Nalbandian A., Sehgal K., Gupta A., Madhavan M. V., McGroder C., Stevens J. S., Cook J. R., Nordvig A. S., Shalev D., Sehrawat T. S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G. F., Bernstein E. J., Mohan S., Beckley A. A., Seres D. S., Choueiri T. K., Uriel N., Ausiello J. C., Accili D., Freedberg D. E., Baldwin M., Schwartz A., Brodie D., Garcia C. K., Elkind M. S. V., Connors J. M., Bilezikian J. P., Landry D. W., Wan E. Y., Post-acute COVID-19 syndrome. Nat. Med. 27, 601–615 (2021). - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

