Safety Monitoring of an Additional Dose of COVID-19 Vaccine - United States, August 12-September 19, 2021
- PMID: 34591835
- PMCID: PMC8486391
- DOI: 10.15585/mmwr.mm7039e4
Safety Monitoring of an Additional Dose of COVID-19 Vaccine - United States, August 12-September 19, 2021
Abstract
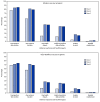
On August 12, 2021, the Food and Drug Administration (FDA) amended Emergency Use Authorizations (EUAs) for the Pfizer-BioNTech and Moderna COVID-19 vaccines to authorize administration of an additional dose after completion of a primary vaccination series to eligible persons with moderate to severe immunocompromising conditions (1,2). On September 22, 2021, FDA authorized an additional dose of Pfizer-BioNTech vaccine ≥6 months after completion of the primary series among persons aged ≥65 years, at high risk for severe COVID-19, or whose occupational or institutional exposure puts them at high risk for COVID-19 (1). Results from a phase 3 clinical trial conducted by Pfizer-BioNTech that included 306 persons aged 18-55 years showed that adverse reactions after receipt of a third dose administered 5-8 months after completion of a 2-dose primary mRNA vaccination series were similar to those reported after receipt of dose 2; these adverse reactions included mild to moderate injection site and systemic reactions (3). CDC developed v-safe, a voluntary, smartphone-based safety surveillance system, to provide information on adverse reactions after COVID-19 vaccination. Coincident with authorization of an additional dose for persons with immunocompromising conditions, the v-safe platform was updated to allow registrants to enter information about additional doses of COVID-19 vaccine received. During August 12-September 19, 2021, a total of 22,191 v-safe registrants reported receipt of an additional dose of COVID-19 vaccine. Most (97.6%) reported a primary 2-dose mRNA vaccination series followed by a third dose of the same vaccine. Among those who completed a health check-in survey for all 3 doses (12,591; 58.1%), 79.4% and 74.1% reported local or systemic reactions, respectively, after dose 3, compared with 77.6% and 76.5% who reported local or systemic reactions, respectively, after dose 2. These initial findings indicate no unexpected patterns of adverse reactions after an additional dose of COVID-19 vaccine; most of these adverse reactions were mild or moderate. CDC will continue to monitor vaccine safety, including the safety of additional doses of COVID-19 vaccine, and provide data to guide vaccine recommendations and protect public health.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine letter of authorization. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2021. https://www.fda.gov/media/150386/download
-
- Food and Drug Administration. Moderna COVID-19 vaccine letter of authorization. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2021. https://www.fda.gov/media/144636/download
-
- Pfizer Inc. Pfizer and BioNTech initiate rolling submission of supplemental biologics license applications to U.S. FDA for booster dose of Comirnaty in individuals 16 and older. New York City, NY: Pfizer Inc.; 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-an...
-
- CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Atlanta, GA: US Department of Health and Human Services, CDC; 2021. Accessed September 25, 2021. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-v...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

