Prevalence of HER2 overexpression and amplification in cervical cancer: A systematic review and meta-analysis
- PMID: 34591928
- PMCID: PMC8483403
- DOI: 10.1371/journal.pone.0257976
Prevalence of HER2 overexpression and amplification in cervical cancer: A systematic review and meta-analysis
Abstract
The reported rates of HER2 positivity in cervical cancer (CC) range from 0% to 87%. The importance of HER2 as an actionable target in CC would depend on HER2 positivity prevalence. Our aim was to provide precise estimates of HER2 overexpression and amplification in CC, globally and by relevant subgroups. We conducted a PRISMA compliant meta-analytic systematic review. We searched Medline, EMBASE, Cochrane database, and grey literature for articles reporting the proportion of HER2 positivity in CC. Studies assessing HER2 status by immunohistochemistry or in situ hybridization in invasive disease were eligible. We performed descriptive analyses of all 65 included studies. Out of these, we selected 26 studies that used standardized American Society of Clinical Oncology / College of American Pathologists (ASCO/CAP) Guidelines compliant methodology. We conducted several meta-analyses of proportions to estimate the pooled prevalence of HER2 positivity and subgroup analyses using geographic region, histology, tumor stage, primary antibody brand, study size, and publication year as moderators. The estimated pooled prevalence of HER2 overexpression was 5.7% (CI 95%: 1.5% to 11.7%) I2 = 87% in ASCO/CAP compliant studies and 27.0%, (CI 95%: 19.9% to 34.8%) I2 = 96% in ASCO/CAP non-compliant ones, p < 0.001. The estimated pooled prevalence of HER2 amplification was 1.2% (CI 95%: 0.0% to 5.8%) I2 = 0% and 24.9% (CI 95%: 12.6% to 39.6%) I2 = 86%, respectively, p = 0.004. No other factor was significantly associated with HER2 positivity rates. Our results suggest that a small, but still meaningful proportion of CC is expected to be HER2-positive. High heterogeneity was the main limitation of the study. Variations in previously reported HER2 positivity rates are mainly related to methodological issues.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

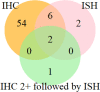







References
-
- World Health Organization. International Agency for Research on Cancer. Cancer Today. [06/08/2021]. Available from: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_.... Accessed 2nd March 2021
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

