Protein disulfide isomerase-A1 regulates intraplatelet reactive oxygen species-thromboxane A2 -dependent pathway in human platelets
- PMID: 34592041
- PMCID: PMC9292974
- DOI: 10.1111/jth.15539
Protein disulfide isomerase-A1 regulates intraplatelet reactive oxygen species-thromboxane A2 -dependent pathway in human platelets
Abstract
Background: Platelet-derived protein disulfide isomerase 1 (PDIA1) regulates thrombus formation, but its role in the regulation of platelet function is not fully understood.
Aims: The aim of this study was to characterize the role of PDIA1 in human platelets.
Methods: Proteomic analysis of PDI isoforms in platelets was performed using liquid chromatography tandem mass spectometry, and the expression of PDIs on platelets in response to collagen, TRAP-14, or ADP was measured with flow cytometry. The effects of bepristat, a selective PDIA1 inhibitor, on platelet aggregation, expression of platelet surface activation markers, thromboxane A2 (TxA2 ), and reactive oxygen species (ROS) generation were evaluated by optical aggregometry, flow cytometry, ELISA, and dihydrodichlorofluorescein diacetate-based fluorescent assay, respectively.
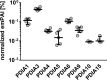
Results: PDIA1 was less abundant compared with PDIA3 in resting platelets and platelets stimulated with TRAP-14, collagen, or ADP. Collagen, but not ADP, induced a significant increase in PDIA1 expression. Bepristat potently inhibited the aggregation of washed platelets induced by collagen or convulxin, but only weakly inhibited platelet aggregation induced by TRAP-14 or thrombin, and had the negligible effect on platelet aggregation induced by arachidonic acid. Inhibition of PDIA1 by bepristat resulted in the reduction of TxA2 and ROS production in collagen- or thrombin-stimulated platelets. Furthermore, bepristat reduced the activation of αIIbβ3 integrin and expression of P-selectin.
Conclusions: PDIA1 acts as an intraplatelet regulator of the ROS-TxA2 pathway in collagen-GP VI receptor-mediated platelet activation that is a mechanistically distinct pathway from extracellular regulation of αIIbβ3 integrin by PDIA3.
Keywords: PDIs expression; bepristat; flow cytometry; platelet activation; proteomics.
© 2021 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
None of authors have a conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

