A cell-type-specific atlas of the inner ear transcriptional response to acoustic trauma
- PMID: 34592158
- PMCID: PMC8709734
- DOI: 10.1016/j.celrep.2021.109758
A cell-type-specific atlas of the inner ear transcriptional response to acoustic trauma
Abstract
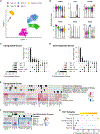
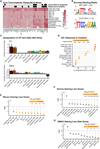
Noise-induced hearing loss (NIHL) results from a complex interplay of damage to the sensory cells of the inner ear, dysfunction of its lateral wall, axonal retraction of type 1C spiral ganglion neurons, and activation of the immune response. We use RiboTag and single-cell RNA sequencing to survey the cell-type-specific molecular landscape of the mouse inner ear before and after noise trauma. We identify induction of the transcription factors STAT3 and IRF7 and immune-related genes across all cell-types. Yet, cell-type-specific transcriptomic changes dominate the response. The ATF3/ATF4 stress-response pathway is robustly induced in the type 1A noise-resilient neurons, potassium transport genes are downregulated in the lateral wall, mRNA metabolism genes are downregulated in outer hair cells, and deafness-associated genes are downregulated in most cell types. This transcriptomic resource is available via the Gene Expression Analysis Resource (gEAR; https://umgear.org/NIHL) and provides a blueprint for the rational development of drugs to prevent and treat NIHL.
Keywords: ATF; IRF7; RiboTag; STAT3; cochlea; noise-induced hearing loss; scRNA-seq; spiral ganglion; transcriptomics.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests K.S.S., J.B.S., G.P., A.T.P., and J.B. are employees of Decibel Therapeutics. The data presented in this manuscript are registered for pending U.S. Provisional Patent Application, number: 63/151,249, title: “System and Methods for Cell Type-Specific Atlas for the Inner Ear Transcriptional Response to Acoustic Trauma,” and UMB docket number: RH-2021-073.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

