TCRαβ/CD19 depleted HSCT from an HLA-haploidentical relative to treat children with different nonmalignant disorders
- PMID: 34592755
- PMCID: PMC8753220
- DOI: 10.1182/bloodadvances.2021005628
TCRαβ/CD19 depleted HSCT from an HLA-haploidentical relative to treat children with different nonmalignant disorders
Abstract
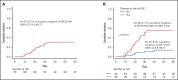
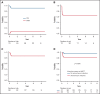
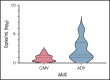
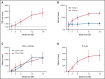
Several nonmalignant disorders (NMDs), either inherited or acquired, can be cured by allogeneic hematopoietic stem cell transplantation (HSCT). Between January 2012 and April 2020, 70 consecutive children affected by primary immunodeficiencies, inherited/acquired bone marrow failure syndromes, red blood cell disorders, or metabolic diseases, lacking a fully matched donor or requiring urgent transplantation underwent TCRαβ/CD19-depleted haploidentical HSCT from an HLA-partially matched relative as part of a prospective study. The median age at transplant was 3.5 years (range 0.3-16.1); the median time from diagnosis to transplant was 10.5 months (2.7 for SCID patients). Primary engraftment was obtained in 51 patients, while 19 and 2 patients experienced either primary or secondary graft failure (GF), the overall incidence of this complication being 30.4%. Most GFs were observed in children with disease at risk for this complication (eg, aplastic anemia, thalassemia). All but 5 patients experiencing GF were successfully retransplanted. Six patients died of infectious complications (4 had active/recent infections at the time of HSCT), the cumulative incidence of transplant-related mortality (TRM) being 8.5%. Cumulative incidence of grade 1-2 acute GVHD was 14.4% (no patient developed grade 3-4 acute GVHD). Only one patient at risk developed mild chronic GVHD. With a median follow-up of 3.5 years, the 5-year probability of overall and disease-free survival was 91.4% and 86.8%, respectively. In conclusion, TCRαβ/CD19-depleted haploidentical HSCT from an HLA-partially matched relative is confirmed to be an effective treatment of children with NMDs. Prompt donor availability, low incidence of GVHD, and TRM make this strategy an attractive option in NMDs patients. The study is registered at ClinicalTrial.gov as NCT01810120.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures


References
-
- Kanate AS, Majhail NS, Savani BN, et al. . Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2020;26(7):1247-1256. - PubMed
-
- Thompson AA, Walters MC, Kwiatkowski J, et al. . Gene therapy in patients with transfusion-dependent β-thalassemia. N Engl J Med. 2018; 378(16):1479-1493. - PubMed
-
- Frangoul H, Altshuler D, Cappellini MD, et al. . CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N Engl J Med. 2021;384(3):252-260. - PubMed
-
- Besse K, Maiers M, Confer D, Albrecht M. On modeling human leukocyte antigen-identical sibling match probability for allogeneic hematopoietic cell transplantation: estimating the need for an unrelated donor source. Biol Blood Marrow Transplant. 2016;22(3):410-417. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

