Loss of glucocorticoid rhythm induces an osteoporotic phenotype in female mice
- PMID: 34592793
- PMCID: PMC8520718
- DOI: 10.1111/acel.13474
Loss of glucocorticoid rhythm induces an osteoporotic phenotype in female mice
Abstract
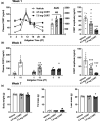
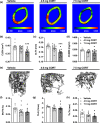
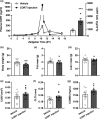
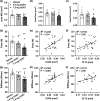
Glucocorticoid (GC)-induced osteoporosis is a widespread health problem that is accompanied with increased fracture risk. Detrimental effects of anti-inflammatory GC therapy on bone have been ascribed to the excess in GC exposure, but it is unknown whether there is also a role for disruption of the endogenous GC rhythm that is inherent to GC therapy. To investigate this, we implanted female C57Bl/6J mice with slow-release corticosterone (CORT) pellets to blunt the rhythm in CORT levels without inducing hypercortisolism. Flattening of CORT rhythm reduced cortical and trabecular bone volume and thickness, whilst bone structure was maintained in mice injected with supraphysiologic CORT at the time of their endogenous GC peak. Mechanistically, mice with a flattened CORT rhythm showed disrupted circadian gene expression patterns in bone, along with changes in circulating bone turnover markers indicative of a negative balance in bone remodelling. Indeed, double calcein labelling of bone in vivo revealed a reduced bone formation in mice with a flattened CORT rhythm. Collectively, these perturbations in bone turnover and structure decreased bone strength and stiffness, as determined by mechanical testing. In conclusion, we demonstrate for the first time that flattening of the GC rhythm disrupts the circadian clock in bone and results in an osteoporotic phenotype in mice. Our findings indicate that at least part of the fracture risk associated with GC therapy may be the consequence of a disturbed GC rhythm, rather than excess GC exposure alone, and that a dampened GC rhythm may contribute to the age-related risk of osteoporosis.
Keywords: bone health; circadian rhythm; corticosteroids; fracture risk; osteoporosis.
© 2021 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Akana, S. F. , Scribner, K. A. , Bradbury, M. J. , Strack, A. M. , Walker, C. D. , & Dallman, M. F. (1992). Feedback sensitivity of the rat hypothalamo‐pituitary‐adrenal axis and its capacity to adjust to exogenous corticosterone. Endocrinology, 131(2), 585–594. 10.1210/endo.131.2.1322275 - DOI - PubMed
-
- Angeli, A. , Guglielmi, G. , Dovio, A. , Capelli, G. , de Feo, D. , Giannini, S. , Giorgino, R. , Moro, L. , & Giustina, A. (2006). High prevalence of asymptomatic vertebral fractures in post‐menopausal women receiving chronic glucocorticoid therapy: a cross‐sectional outpatient study. Bone, 39(2), 253–259. 10.1016/j.bone.2006.02.005 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

