Loss of presenilin function enhances tau phosphorylation and aggregation in mice
- PMID: 34593029
- PMCID: PMC8482568
- DOI: 10.1186/s40478-021-01259-7
Loss of presenilin function enhances tau phosphorylation and aggregation in mice
Abstract
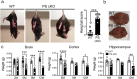
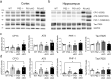
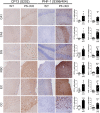
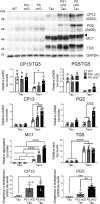
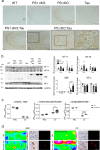
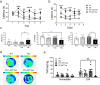
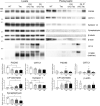
Mutations in the presenilin (PS/PSEN) genes encoding the catalytic components of γ-secretase accelerate amyloid-β (Aβ) and tau pathologies in familial Alzheimer's disease (AD). Although the mechanisms by which these mutations affect Aβ are well defined, the precise role PS/γ-secretase on tau pathology in neurodegeneration independently of Aβ is largely unclear. Here we report that neuronal PS deficiency in conditional knockout (cKO) mice results in age-dependent brain atrophy, inflammatory responses and accumulation of pathological tau in neurons and glial cells. Interestingly, genetic inactivation of presenilin 1 (PS1) or both PS genes in mutant human Tau transgenic mice exacerbates memory deficits by accelerating phosphorylation and aggregation of tau in excitatory neurons of vulnerable AD brain regions (e.g., hippocampus, cortex and amygdala). Remarkably, neurofilament (NF) light chain (NF-L) and phosphorylated NF are abnormally accumulated in the brain of Tau mice lacking PS. Synchrotron infrared microspectroscopy revealed aggregated and oligomeric β-sheet structures in amyloid plaque-free PS-deficient Tau mice. Hippocampal-dependent memory deficits are associated with synaptic tau accumulation and reduction of pre- and post-synaptic proteins in Tau mice. Thus, partial loss of PS/γ-secretase in neurons results in temporal- and spatial-dependent tau aggregation associated with memory deficits and neurodegeneration. Our findings show that tau phosphorylation and aggregation are key pathological processes that may underlie neurodegeneration caused by familial AD-linked PSEN mutations.
Keywords: Alzheimer’s disease; Memory; Neurofilament; Presenilin; Synapse; Tau; β-sheet aggregation; γ-secretase.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no financial competing conflicts of interest in relation to this work.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

