Post-discharge after surgery Virtual Care with Remote Automated Monitoring-1 (PVC-RAM-1) technology versus standard care: randomised controlled trial
- PMID: 34593374
- PMCID: PMC8477638
- DOI: 10.1136/bmj.n2209
Post-discharge after surgery Virtual Care with Remote Automated Monitoring-1 (PVC-RAM-1) technology versus standard care: randomised controlled trial
Abstract
Objective: To determine if virtual care with remote automated monitoring (RAM) technology versus standard care increases days alive at home among adults discharged after non-elective surgery during the covid-19 pandemic.
Design: Multicentre randomised controlled trial.
Setting: 8 acute care hospitals in Canada.
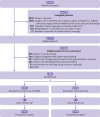
Participants: 905 adults (≥40 years) who resided in areas with mobile phone coverage and were to be discharged from hospital after non-elective surgery were randomised either to virtual care and RAM (n=451) or to standard care (n=454). 903 participants (99.8%) completed the 31 day follow-up.
Intervention: Participants in the experimental group received a tablet computer and RAM technology that measured blood pressure, heart rate, respiratory rate, oxygen saturation, temperature, and body weight. For 30 days the participants took daily biophysical measurements and photographs of their wound and interacted with nurses virtually. Participants in the standard care group received post-hospital discharge management according to the centre's usual care. Patients, healthcare providers, and data collectors were aware of patients' group allocations. Outcome adjudicators were blinded to group allocation.
Main outcome measures: The primary outcome was days alive at home during 31 days of follow-up. The 12 secondary outcomes included acute hospital care, detection and correction of drug errors, and pain at 7, 15, and 30 days after randomisation.
Results: All 905 participants (mean age 63.1 years) were analysed in the groups to which they were randomised. Days alive at home during 31 days of follow-up were 29.7 in the virtual care group and 29.5 in the standard care group: relative risk 1.01 (95% confidence interval 0.99 to 1.02); absolute difference 0.2% (95% confidence interval -0.5% to 0.9%). 99 participants (22.0%) in the virtual care group and 124 (27.3%) in the standard care group required acute hospital care: relative risk 0.80 (0.64 to 1.01); absolute difference 5.3% (-0.3% to 10.9%). More participants in the virtual care group than standard care group had a drug error detected (134 (29.7%) v 25 (5.5%); absolute difference 24.2%, 19.5% to 28.9%) and a drug error corrected (absolute difference 24.4%, 19.9% to 28.9%). Fewer participants in the virtual care group than standard care group reported pain at 7, 15, and 30 days after randomisation: absolute differences 13.9% (7.4% to 20.4%), 11.9% (5.1% to 18.7%), and 9.6% (2.9% to 16.3%), respectively. Beneficial effects proved substantially larger in centres with a higher rate of care escalation.
Conclusion: Virtual care with RAM shows promise in improving outcomes important to patients and to optimal health system function.
Trial registration: ClinicalTrials.gov NCT04344665.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare: support from Roche, McMaster University, the Research Institute of St Joseph’s Healthcare Hamilton, Ottawa Hospital Academic Medical Association, Queen’s University, Hamilton Health Sciences, Kingston Health Sciences, London Health Sciences, St Joseph’s Healthcare Hamilton, the Ottawa Hospital, and the University of Alberta Hospital for the submitted work; a financial relationship with Cloud Diagnostics Canada for purchase of devices and data plans used in this trial; and a relationship with Cloud Diagnostics Canada, which undertook training sessions for virtual nurses and perioperative doctors and surgeons on how to use their technology.
Figures

References
-
- Canadian Institutes of Health Information. All-cause readmission to acute care and return to the emergency department, 2012. https://publications.gc.ca/collections/collection_2013/icis-cihi/H118-93...
-
- Perakslis E, Ginsburg GS. Digital Health-The Need to Assess Benefits, Risks, and Value. JAMA 2021;325:127-8. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
