Frequency-Dependent Action of Neuromodulation
- PMID: 34593519
- PMCID: PMC8584230
- DOI: 10.1523/ENEURO.0338-21.2021
Frequency-Dependent Action of Neuromodulation
Abstract
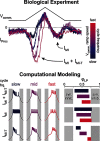
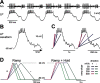
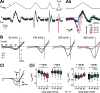
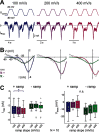
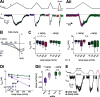
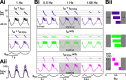
In oscillatory circuits, some actions of neuromodulators depend on the oscillation frequency. However, the mechanisms are poorly understood. We explored this problem by characterizing neuromodulation of the lateral pyloric (LP) neuron of the crab stomatogastric ganglion (STG). Many peptide modulators, including proctolin, activate the same ionic current (IMI) in STG neurons. Because IMI is fast and non-inactivating, its peak level does not depend on the temporal properties of neuronal activity. We found, however, that the amplitude and peak time of the proctolin-activated current in LP is frequency dependent. Because frequency affects the rate of voltage change, we measured these currents with voltage ramps of different slopes and found that proctolin activated two kinetically distinct ionic currents: the known IMI, whose amplitude is independent of ramp slope or direction, and an inactivating current (IMI-T), which was only activated by positive ramps and whose amplitude increased with increasing ramp slope. Using a conductance-based model we found that IMI and IMI-T make distinct contributions to the bursting activity, with IMI increasing the excitability, and IMI-T regulating the burst onset by modifying the postinhibitory rebound in a frequency-dependent manner. The voltage dependence and partial calcium permeability of IMI-T is similar to other characterized neuromodulator-activated currents in this system, suggesting that these are isoforms of the same channel. Our computational model suggests that calcium permeability may allow this current to also activate the large calcium-dependent potassium current in LP, providing an additional mechanism to regulate burst termination. These results demonstrate a mechanism for frequency-dependent actions of neuromodulators.
Keywords: calcium; central pattern generator; modeling; neuromodulation; stomatogastric.
Copyright © 2021 Schneider et al.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
