Gut microbiota-derived short-chain fatty acids protect against the progression of endometriosis
- PMID: 34593556
- PMCID: PMC8500332
- DOI: 10.26508/lsa.202101224
Gut microbiota-derived short-chain fatty acids protect against the progression of endometriosis
Abstract
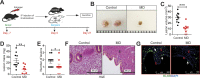
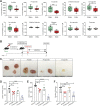
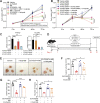
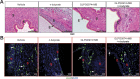
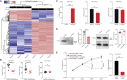
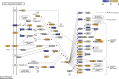
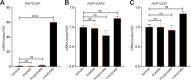
Worldwide, ∼196 million are afflicted with endometriosis, a painful disease in which endometrial tissue implants and proliferates on abdominal peritoneal surfaces. Theories on the origin of endometriosis remained inconclusive. Whereas up to 90% of women experience retrograde menstruation, only 10% develop endometriosis, suggesting that factors that alter peritoneal environment might contribute to endometriosis. Herein, we report that whereas some gut bacteria promote endometriosis, others protect against endometriosis by fermenting fiber to produce short-chain fatty acids. Specifically, we found that altered gut microbiota drives endometriotic lesion growth and feces from mice with endometriosis contained less of short-chain fatty acid and n-butyrate than feces from mice without endometriosis. Treatment with n-butyrate reduced growth of both mouse endometriotic lesions and human endometriotic lesions in a pre-clinical mouse model. Mechanistic studies revealed that n-butyrate inhibited human endometriotic cell survival and lesion growth through G-protein-coupled receptors, histone deacetylases, and a GTPase activating protein, RAP1GAP. Our findings will enable future studies aimed at developing diagnostic tests, gut bacteria metabolites and treatment strategies, dietary supplements, n-butyrate analogs, or probiotics for endometriosis.
© 2021 Chadchan et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
