A novel thermostable prokaryotic fucoidan active sulfatase PsFucS1 with an unusual quaternary hexameric structure
- PMID: 34593864
- PMCID: PMC8484680
- DOI: 10.1038/s41598-021-98588-3
A novel thermostable prokaryotic fucoidan active sulfatase PsFucS1 with an unusual quaternary hexameric structure
Abstract
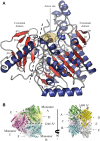
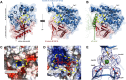
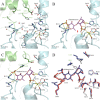
Fucoidans are sulfated, fucose-rich marine polysaccharides primarily found in cell walls of brown seaweeds (macroalgae). Fucoidans are known to possess beneficial bioactivities depending on their structure and sulfation degree. Here, we report the first functional characterization and the first crystal structure of a prokaryotic sulfatase, PsFucS1, belonging to sulfatase subfamily S1_13, able to release sulfate from fucoidan oligosaccharides. PsFucS1 was identified in the genome of a Pseudoalteromonas sp. isolated from sea cucumber gut. PsFucS1 (57 kDa) is Ca2+ dependent and has an unusually high optimal temperature (68 °C) and thermostability. Further, the PsFucS1 displays a unique quaternary hexameric structure comprising a tight trimeric dimer complex. The structural data imply that this hexamer formation results from an uncommon interaction of each PsFucS1 monomer that is oriented perpendicular to the common dimer interface (~ 1500 Å2) that can be found in analogous sulfatases. The uncommon interaction involves interfacing (1246 Å2) through a bundle of α-helices in the N-terminal domain to form a trimeric ring structure. The high thermostability may be related to this unusual quaternary hexameric structure formation that is suggested to represent a novel protein thermostabilization mechanism.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Torres MD, et al. Fucoidans: The importance of processing on their anti-tumoral properties. Algal Res. 2020;45:101748. doi: 10.1016/j.algal.2019.101748. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

