Neuronal let-7b-5p acts through the Hippo-YAP pathway in neonatal encephalopathy
- PMID: 34593980
- PMCID: PMC8484486
- DOI: 10.1038/s42003-021-02672-3
Neuronal let-7b-5p acts through the Hippo-YAP pathway in neonatal encephalopathy
Abstract
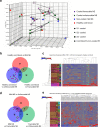
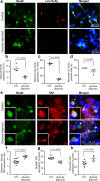
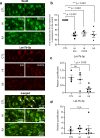
Despite increasing knowledge on microRNAs, their role in the pathogenesis of neonatal encephalopathy remains to be elucidated. Herein, we identify let-7b-5p as a significant microRNA in neonates with moderate to severe encephalopathy from dried blood spots using next generation sequencing. Validation studies using Reverse Transcription and quantitative Polymerase Chain Reaction on 45 neonates showed that let-7b-5p expression was increased on day 1 in neonates with moderate to severe encephalopathy with unfavourable outcome when compared to those with mild encephalopathy. Mechanistic studies performed on glucose deprived cell cultures and the cerebral cortex of two animal models of perinatal brain injury, namely hypoxic-ischaemic and intrauterine inflammation models confirm that let-7b-5p is associated with the apoptotic Hippo pathway. Significant reduction in neuronal let-7b-5p expression corresponded with activated Hippo pathway, with increased neuronal/nuclear ratio of Yes Associated Protein (YAP) and increased neuronal cleaved caspase-3 expression in both animal models. Similar results were noted for let-7b-5p and YAP expression in glucose-deprived cell cultures. Reduced nuclear YAP with decreased intracellular let-7b-5p correlated with neuronal apoptosis in conditions of metabolic stress. This finding of the Hippo-YAP association with let-7b needs validation in larger cohorts to further our knowledge on let-7b-5p as a biomarker for neonatal encephalopathy.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

