Increased somatic mutation burdens in normal human cells due to defective DNA polymerases
- PMID: 34594041
- PMCID: PMC8492474
- DOI: 10.1038/s41588-021-00930-y
Increased somatic mutation burdens in normal human cells due to defective DNA polymerases
Abstract
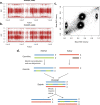
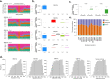
Mutation accumulation in somatic cells contributes to cancer development and is proposed as a cause of aging. DNA polymerases Pol ε and Pol δ replicate DNA during cell division. However, in some cancers, defective proofreading due to acquired POLE/POLD1 exonuclease domain mutations causes markedly elevated somatic mutation burdens with distinctive mutational signatures. Germline POLE/POLD1 mutations cause familial cancer predisposition. Here, we sequenced normal tissue and tumor DNA from individuals with germline POLE/POLD1 mutations. Increased mutation burdens with characteristic mutational signatures were found in normal adult somatic cell types, during early embryogenesis and in sperm. Thus human physiology can tolerate ubiquitously elevated mutation burdens. Except for increased cancer risk, individuals with germline POLE/POLD1 mutations do not exhibit overt features of premature aging. These results do not support a model in which all features of aging are attributable to widespread cell malfunction directly resulting from somatic mutation burdens accrued during life.
© 2021. The Author(s).
Conflict of interest statement
P.J.C. is a founder, consultant and stockholder of Mu Genomics Ltd. The other authors declare no competing interests.
Figures











References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

