Short-Term Evaluation of Dupilumab Effects in Patients with Severe Asthma and Nasal Polyposis
- PMID: 34594115
- PMCID: PMC8478424
- DOI: 10.2147/JAA.S328988
Short-Term Evaluation of Dupilumab Effects in Patients with Severe Asthma and Nasal Polyposis
Abstract
Background: Having been approved for biological treatment of atopic dermatitis, dupilumab has also been recently licensed as add-on therapy for severe asthma and nasal polyposis. With regard to the latter diseases, few real-life clinical investigations have been carried out to date.
Objective: The primary end point of this single-center observational study was to evaluate in a real-life setting the short-term therapeutic effects of dupilumab in patients with severe asthma and nasal polyposis.
Methods: At baseline and after 4 weeks of add-on therapy with dupilumab, several clinical and functional parameters were assessed in 20 patients with severe asthma and nasal polyposis, including both allergic and nonallergic subjects.
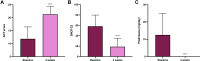
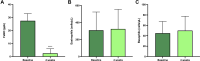
Results: After 4 weeks of treatment with dupilumab, all patients experienced remarkable improvement in both severe asthma and nasal polyposis. In particular, asthma-control test and sinonasal outcome test 22 scores had significantly increased (p<0.0001) and decreased (p<0.0001), respectively. Oral corticosteroid intake got to zero within 4 weeks (p<0.0001). Moreover, in week 4, significant increases were detected with regard to both prebronchodilator forced expiratory volume in the first second (p<0.01) and forced vital capacity (FVC; p<0.05). At the same time point, dupilumab had significantly reduced residual volume (p<0.0001) and total lung capacity (p<0.001), whereas it had enhanced forced midexpiratory flow of 25%-75% FVC (p<0.01) and peak expiratory flow (p<0.01). After 4 weeks of treatment, dupilumab had also lowered levels of fractional exhaled nitric oxide (p<0.0001).
Conclusion: The results of this real-life study suggest that dupilumab can be utilized in both allergic and nonallergic patients with severe asthma and nasal polyposis as a valuable add-on biological therapy with rapid onset of action.
Keywords: dupilumab; interleukin 4 receptor; nasal polyposis; severe asthma.
© 2021 Pelaia et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest in this work.
Figures

References
LinkOut - more resources
Full Text Sources

