Weak Vestibular Response in Persistent Developmental Stuttering
- PMID: 34594189
- PMCID: PMC8477904
- DOI: 10.3389/fnint.2021.662127
Weak Vestibular Response in Persistent Developmental Stuttering
Abstract
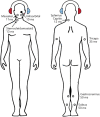
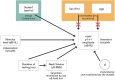
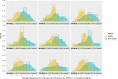
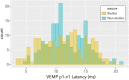
Vibrational energy created at the larynx during speech will deflect vestibular mechanoreceptors in humans (Todd et al., 2008; Curthoys, 2017; Curthoys et al., 2019). Vestibular-evoked myogenic potential (VEMP), an indirect measure of vestibular function, was assessed in 15 participants who stutter, with a non-stutter control group of 15 participants paired on age and sex. VEMP amplitude was 8.5 dB smaller in the stutter group than the non-stutter group (p = 0.035, 95% CI [-0.9, -16.1], t = -2.1, d = -0.8, conditional R 2 = 0.88). The finding is subclinical as regards gravitoinertial function, and is interpreted with regard to speech-motor function in stuttering. There is overlap between brain areas receiving vestibular innervation, and brain areas identified as important in studies of persistent developmental stuttering. These include the auditory brainstem, cerebellar vermis, and the temporo-parietal junction. The finding supports the disruptive rhythm hypothesis (Howell et al., 1983; Howell, 2004) in which sensory inputs additional to own speech audition are fluency-enhancing when they coordinate with ongoing speech.
Keywords: VEMP; own voice identification; speech perception; speech-motor control; stuttering; vestibular.
Copyright © 2021 Gattie, Lieven and Kluk.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










Similar articles
-
Verbal dichotic listening in developmental stuttering: subgroups with atypical auditory processing.Cogn Behav Neurol. 2004 Dec;17(4):224-32. Cogn Behav Neurol. 2004. PMID: 15622019 Clinical Trial.
-
Left posterior-dorsal area 44 couples with parietal areas to promote speech fluency, while right area 44 activity promotes the stopping of motor responses.Neuroimage. 2016 Nov 15;142:628-644. doi: 10.1016/j.neuroimage.2016.08.030. Epub 2016 Aug 16. Neuroimage. 2016. PMID: 27542724
-
Limited Pre-Speech Auditory Modulation in Individuals Who Stutter: Data and Hypotheses.J Speech Lang Hear Res. 2019 Aug 29;62(8S):3071-3084. doi: 10.1044/2019_JSLHR-S-CSMC7-18-0358. Epub 2019 Aug 29. J Speech Lang Hear Res. 2019. PMID: 31465711 Free PMC article.
-
Common features of fluency-evoking conditions studied in stuttering subjects and controls: an H(2)15O PET study.J Fluency Disord. 2003 Winter;28(4):319-35; quiz 336. doi: 10.1016/j.jfludis.2003.08.004. J Fluency Disord. 2003. PMID: 14643068 Review.
-
The Role of Sensory Feedback in Developmental Stuttering: A Review.Neurobiol Lang (Camb). 2021 Jun 9;2(2):308-334. doi: 10.1162/nol_a_00036. eCollection 2021. Neurobiol Lang (Camb). 2021. PMID: 37216145 Free PMC article. Review.
Cited by
-
Reduced Vestibular Function is Associated With Cortical Surface Shape Changes in the Frontal Cortex.Hum Brain Mapp. 2025 Jul;46(10):e70251. doi: 10.1002/hbm.70251. Hum Brain Mapp. 2025. PMID: 40631647 Free PMC article.
-
Novel cVEMP procedure reveals sexual dimorphism in peak to trough latency.Front Integr Neurosci. 2025 Apr 9;19:1454924. doi: 10.3389/fnint.2025.1454924. eCollection 2025. Front Integr Neurosci. 2025. PMID: 40271199 Free PMC article.
-
Reduced Vestibular Function is Associated with Cortical Surface Shape Changes in the Frontal Cortex.medRxiv [Preprint]. 2024 Nov 23:2024.11.22.24317807. doi: 10.1101/2024.11.22.24317807. medRxiv. 2024. Update in: Hum Brain Mapp. 2025 Jul;46(10):e70251. doi: 10.1002/hbm.70251. PMID: 39606396 Free PMC article. Updated. Preprint.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

