TFEC contributes to cardiac hypertrophy by inhibiting AMPK/mTOR signaling
- PMID: 34594408
- PMCID: PMC8456502
- DOI: 10.3892/etm.2021.10706
TFEC contributes to cardiac hypertrophy by inhibiting AMPK/mTOR signaling
Abstract
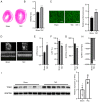
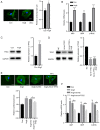
The underlying mechanism of cardiac hypertrophy has not yet been fully elucidated. The present study aimed to explore the function of transcription factor EC (TFEC) in mouse models of cardiac hypertrophy and to determine the underlying mechanism. Pressure-overload cardiac hypertrophy and angiotensin II (AngII) infusion-induced animal models of cardiac hypertrophy were established in vivo. The expression of TFEC was explored via western blotting. The results demonstrated that TFEC expression was significantly increased in the hearts of mice with pressure overload- and AngII-induced hypertrophy. Injection of rAd-short hairpin (sh)-TFEC significantly decreased the expression of TFEC in heart tissues compared with group injected with rAd-negative control (NC). Furthermore, the expression levels of atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and β-myosin heavy chain (β-MHC) were increased in the hearts of AngII-treated mice; however, compared with rAd-NC transfection, transfection with rAd-sh-TFEC decreased the expression levels of ANP, BNP and β-MHC. The results from echocardiographic analysis indicated that transfection with rAd-sh-TFEC improved the cardiac function of AngII-treated mice compared with transfection with rAd-NC. In addition, the AngII-induced increase in cardiomyocyte size could be reversed by TFEC knockdown in primary cardiomyocytes. The elevated expression levels of ANP, BNP and β-MHC induced by AngII could be partially abolished following TFEC knockdown. The results from western blotting demonstrated that TFEC overexpression decreased the expression of phosphorylated AMP-activated protein kinase (AMPK)/acetyl-CoA carboxylase (ACC) but increased the expression of phosphorylated mechanistic target of rapamycin (mTOR). Furthermore, Compound C significantly suppressed the activation of AMPK/ACC but increased the activation of mTOR, even in primary cardiomyocytes transfected with rAd-sh-TFEC. In conclusion, the findings from this study demonstrated that TFEC was overexpressed in the hearts of mice with cardiac hypertrophy and that silencing TFEC may improve AngII-induced cardiac hypertrophy and dysfunction by activating AMPK/mTOR signaling.
Keywords: AMP-activated protein kinase/mechanistic target of rapamycin signaling; cardiac hypertrophy; kinase non-catalytic C-lobe domain containing 1.
Copyright: © Zhao et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
