Ovarian Function Suppression With Luteinizing Hormone-Releasing Hormone Agonists for the Treatment of Hormone Receptor-Positive Early Breast Cancer in Premenopausal Women
- PMID: 34595110
- PMCID: PMC8477635
- DOI: 10.3389/fonc.2021.700722
Ovarian Function Suppression With Luteinizing Hormone-Releasing Hormone Agonists for the Treatment of Hormone Receptor-Positive Early Breast Cancer in Premenopausal Women
Abstract
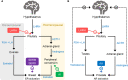
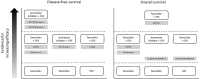
Chemotherapy and endocrine therapies are mainstays of treatment for early and advanced hormone receptor-positive (HR+) breast cancer. In premenopausal women with HR+ tumors, the benefits of adding ovarian function suppression (OFS) to endocrine therapy have been debated. Consequently, for many years, tamoxifen monotherapy has been the standard of care for endocrine treatment in the adjuvant setting. Recent studies have, however, provided new evidence that, in some premenopausal patients, OFS in combination with tamoxifen or aromatase inhibitors (AIs) can significantly increase survival versus tamoxifen alone. Luteinizing hormone-releasing hormone agonists (LHRHa), including goserelin, triptorelin, and leuprorelin, achieve OFS through sustained suppression of the release of follicle-stimulating hormone and luteinizing hormone from the pituitary. In turn, this suppresses production and secretion of estradiol, an ovarian hormone that supports cancer cell growth, survival, and proliferation. In this review, we discuss the clinical evidence supporting the addition of LHRHa to adjuvant endocrine therapies, including tamoxifen and AIs, for premenopausal women with breast cancer. We also discuss the role of LHRHa use in combination with adjuvant chemotherapy to preserve ovarian function and fertility in young patients with breast cancer. Finally, we discuss important practical aspects of the use of LHRHa in breast cancer treatment, including side-effects, patient adherence to treatment, and the use of slow-release, long-acting drug formulations.
Keywords: breast cancer; endocrine therapy; luteinizing hormone releasing hormone; ovarian function preservation; ovarian function suppression (OFS); premenopausal.
Copyright © 2021 Lu, Wong and Kim.
Conflict of interest statement
Authors received no financial compensation for this work. Y-SL has received personal fees from AstraZeneca, Eisai, EuroPharma and personal fees, research support, and other financial support from Novartis, Roche, Eli Lilly, Merck, and Pfizer. AW has received personal fees from AstraZeneca and Pfizer and research support from Otsuka Pharmaceuticals. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- World Health Organization . Cancer Fact Sheet. World Health Organization; (2018). Geneva, Switzerland. Available at: https://www.who.int/news-room/fact-sheets/detail/cancer.
Publication types
LinkOut - more resources
Full Text Sources
Medical

