Endovascular treatment of femoro-popliteal disease with the Supera stent: results of a multicenter study
- PMID: 34595901
- PMCID: PMC8847959
- DOI: 10.4081/jphr.2021.2360
Endovascular treatment of femoro-popliteal disease with the Supera stent: results of a multicenter study
Abstract
Background: Even though many types of stents have been tested in superficial femoral artery (SFA) and popliteal artery (PA), most of these devices have provided an unsatisfactory outcome, probably due their unsuitable anatomical and physiological characteristics. The Supera peripheral stent (Abbott Vascular, Santa Rosa, CA, USA) is a braided interwoven nitinol device specifically designed for treating atherosclerotic lesions of the femoro-popliteal segment. The aim of this multicenter retrospective study was to describe the effectiveness of Supera stents in the management of femoral-popliteal atherosclerotic lesions and to critically analyze our findings in the context of current and past literature.
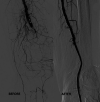
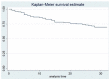
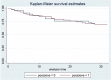
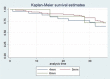
Design and methods: In this study we enrolled only patients who satisfied the inclusion criteria: i) patients affected by chronic obstructive arterial disease (COAD) grade II, as per Rutherford classification; ii) patients treated with endovascular revascularization and Supera stent implantation in the femoro-popliteal axis. We retrospectively analyzed the Doppler Ultra-sound (US) follow-up at 12-24 and 36 months to detect the vascular occlusions. The primary patency, primary patency assisted and TLR were described statistically analyzed by survival analysis and the demographic data, clinical data, device safety following stenting were described as frequency and mean value.
Results: 105 endovascular procedures on 99 patients for femoro-popliteal stenting with Supera were performed in four Italian hospitals. The median follow-up was 39 months (range 6-72), with primary patency rate of 83.1%, 74.3% and 69.5% at 12, 24 and 36 months after the procedure. The primary patency assisted was 89.9%, 76.8% and 73.4% in the same period, while the freedom from TLR values were 92.7%, 91.5% and 89.5% at 12, 24 and 36 months after the procedure, respectively. The mortality rate recorded at 12 months from the Supera implantation was 2.8% (3 out of 99 patients enrolled).
Conclusions: Our data were in agreement with the current literature, showing the non-inferiority Supera stent in relation to the other stent available. Supera stent showed an excellent safety, effectiveness profile and high durability for the treatment of PAD patients with femoro-popliteal artery disease.
Figures

References
-
- Zeller T. Current state of endovascular treatment of femoropopliteal artery disease. Vasc Med 2007;12:223-34. - PubMed
-
- de Boer SW, van den Heuvel DAF, de Vries-Werson DAB, et al. . Short-term results of the RAPID randomized trial of the legflow paclitaxel-eluting balloon with Supera stenting vs Supera stenting alone for the treatment of intermediate and long superficial femoral artery lesions. J Endovasc Ther 2017;24:783-92. - PubMed
-
- Chan YC, Cheng SW, Cheung GC. A midterm analysis of patients who received femoropoplitel helical interwoven nitinol stents. J Vasc Surg 2020;71:2048-55. - PubMed
-
- Gray WA, Feiring A, Cioppi M, et al. . S.M.A.R.T. self-expanding nitinol stent for the treatment of atherosclerotic lesions in the superficial femoral artery (STROLL): 1-year outcomes. J Vasc Interv Radiol 2015;26:21-8. - PubMed
-
- Fowkes FGR, Rudan D, Rudan I, et al. . Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013;382:1329-40. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

