Defining circadian disruption in neurodegenerative disorders
- PMID: 34596047
- PMCID: PMC8483739
- DOI: 10.1172/JCI148288
Defining circadian disruption in neurodegenerative disorders
Abstract
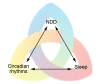
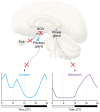
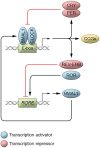
Neurodegenerative diseases encompass a large group of conditions that are clinically and pathologically diverse yet are linked by a shared pathology of misfolded proteins. The accumulation of insoluble aggregates is accompanied by a progressive loss of vulnerable neurons. For some patients, the symptoms are motor focused (ataxias), while others experience cognitive and psychiatric symptoms (dementias). Among the shared symptoms of neurodegenerative diseases is a disruption of the sleep/wake cycle that occurs early in the trajectory of the disease and may be a risk factor for disease development. In many cases, the disruption in the timing of sleep and other rhythmic physiological markers immediately raises the possibility of neurodegeneration-driven disruption of the circadian timing system. The aim of this Review is to summarize the evidence supporting the hypothesis that circadian disruption is a core symptom within neurodegenerative diseases, including Alzheimer's disease, Huntington's disease, and Parkinson's disease, and to discuss the latest progress in this field. The Review discusses evidence that neurodegenerative processes may disrupt the structure and function of the circadian system and describes circadian-based interventions as well as timed drug treatments that may improve a wide range of symptoms associated with neurodegenerative disorders. It also identifies key gaps in our knowledge.
Conflict of interest statement
Figures