Pathogen-Specific Effects of Probiotics in Children With Acute Gastroenteritis Seeking Emergency Care: A Randomized Trial
- PMID: 34596225
- PMCID: PMC9402642
- DOI: 10.1093/cid/ciab876
Pathogen-Specific Effects of Probiotics in Children With Acute Gastroenteritis Seeking Emergency Care: A Randomized Trial
Abstract
Background: It is unknown if probiotics exert pathogen-specific effects in children with diarrhea secondary to acute gastroenteritis.
Methods: Analysis of patient-level data from 2 multicenter randomized, placebo controlled trials conducted in pediatric emergency departments in Canada and the United States. Participants were 3-48 months with >3 diarrheal episodes in the preceding 24 hours and were symptomatic for <72 hours and <7 days in the Canadian and US studies, respectively. Participants received either placebo or a probiotic preparation (Canada-Lactobacillus rhamnosus R0011/Lactobacillus helveticus R0052; US-L. rhamnosus GG). The primary outcome was post-intervention moderate-to-severe disease (ie, ≥9 on the Modified Vesikari Scale [MVS] score).
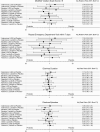
Results: Pathogens were identified in specimens from 59.3% of children (928/1565). No pathogen groups were less likely to experience an MVS score ≥9 based on treatment allocation (test for interaction = 0.35). No differences between groups were identified for adenovirus (adjusted relative risk [aRR]: 1.42; 95% confidence interval [CI]: .62, 3.23), norovirus (aRR: 0.98; 95% CI: .56, 1.74), rotavirus (aRR: 0.86; 95% CI: .43, 1.71) or bacteria (aRR: 1.19; 95% CI: .41, 3.43). At pathogen-group and among individual pathogens there were no differences in diarrhea duration or the total number of diarrheal stools between treatment groups, regardless of intervention allocation or among probiotic sub-groups. Among adenovirus-infected children, those administered the L. rhamnosus R0011/L. helveticus R0052 product experienced fewer diarrheal episodes (aRR: 0.65; 95% CI: .47, .90).
Conclusions: Neither probiotic product resulted in less severe disease compared to placebo across a range of the most common etiologic pathogens. The preponderance of evidence does not support the notion that there are pathogen specific benefits associated with probiotic use in children with acute gastroenteritis.
Clinical trials registration: NCT01773967 and NCT01853124.
Keywords: child; diarrhea; emergency service; gastroenteritis; hospital; probiotic.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

References
-
- Wilkins T, Sequoia J.. Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician 2017; 96:170–8. - PubMed
-
- Szajewska H, Kołodziej M, Gieruszczak-Białek D, Skórka A, Ruszczyński M, Shamir R.. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children: a 2019 update. Aliment Pharmacol Ther 2019; 49:1376–84. - PubMed
-
- Guarino A, Guandalini S, Lo Vecchio A.. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol 2015; 49 Suppl 1:S37–45. - PubMed
-
- Freedman SB, Williamson-Urquhart S, Farion KJ, et al. ; PERC PROGUT Trial Group. . Multicenter trial of a combination probiotic for children with gastroenteritis. N Engl J Med 2018; 379:2015–26. - PubMed

