Extracellular vesicles from adipose tissue-derived stem cells alleviate osteoporosis through osteoprotegerin and miR-21-5p
- PMID: 34596354
- PMCID: PMC8485335
- DOI: 10.1002/jev2.12152
Extracellular vesicles from adipose tissue-derived stem cells alleviate osteoporosis through osteoprotegerin and miR-21-5p
Abstract
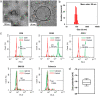
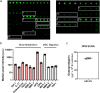
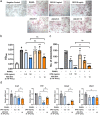
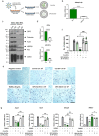
Osteoporosis is one of the most common skeletal disorders caused by the imbalance between bone formation and resorption, resulting in quantitative loss of bone tissue. Since stem cell-derived extracellular vesicles (EVs) are growing attention as novel cell-free therapeutics that have advantages over parental stem cells, the therapeutic effects of EVs from adipose tissue-derived stem cells (ASC-EVs) on osteoporosis pathogenesis were investigated. ASC-EVs were isolated by a multi-filtration system based on the tangential flow filtration (TFF) system and characterized using transmission electron microscopy, dynamic light scattering, zeta potential, flow cytometry, cytokine arrays, and enzyme-linked immunosorbent assay. EVs are rich in growth factors and cytokines related to bone metabolism and mesenchymal stem cell (MSC) migration. In particular, osteoprotegerin (OPG), a natural inhibitor of receptor activator of nuclear factor-κB ligand (RANKL), was highly enriched in ASC-EVs. We found that the intravenous administration of ASC-EVs attenuated bone loss in osteoporosis mice. Also, ASC-EVs significantly inhibited osteoclast differentiation of macrophages and promoted the migration of bone marrow-derived MSCs (BM-MSCs). However, OPG-depleted ASC-EVs did not show anti-osteoclastogenesis effects, demonstrating that OPG is critical for the therapeutic effects of ASC-EVs. Additionally, small RNA sequencing data were analysed to identify miRNA candidates related to anti-osteoporosis effects. miR-21-5p in ASC-EVs inhibited osteoclast differentiation through Acvr2a down-regulation. Also, let-7b-5p in ASC-EVs significantly reduced the expression of genes related to osteoclastogenesis. Finally, ASC-EVs reached the bone tissue after they were injected intravenously, and they remained longer. OPG, miR-21-5p, and let-7b-5p in ASC-EVs inhibit osteoclast differentiation and reduce gene expression related to bone resorption, suggesting that ASC-EVs are highly promising as cell-free therapeutic agents for osteoporosis treatment.
Keywords: extracellular vesicles; human adipose tissue-derived stem cells; let-7b-5p; miR-21-5p; osteoporosis; osteoprotegerin.
© 2021 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
J.H.P., Y.W.C., and D.G.J. are stockholders of Exostemtech, Inc. J.S.C., K.S.L., S.H.Y., C.H.W., Y.J.J., and S.Y.P. are employed by Exostemtech, Inc. The other authors have no conflicts of interest to declare.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

