Mechanochemical Synthesis of Primary Amides
- PMID: 34596412
- PMCID: PMC8524419
- DOI: 10.1021/acs.joc.1c02350
Mechanochemical Synthesis of Primary Amides
Abstract
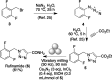
Ball milling of aromatic, heteroaromatic, vinylic, and aliphatic esters with ethanol and calcium nitride afforded the corresponding primary amides in a transformation that was compatible with a variety of functional groups and maintained the integrity of a stereocenter α to carbonyl. This methodology was applied to α-amino esters and N-BOC dipeptide esters and also to the synthesis of rufinamide, an antiepileptic drug.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
-
For selected recent reviews, see:
- O’Neill R. T.; Boulatov R. The many flavours of mechanochemistry and its plausible conceptual underpinnings. Nat. Chem. Rev. 2021, 5, 148–167. 10.1038/s41570-020-00249-y. - DOI - PubMed
- Egorov I. N.; Santra S.; Kopchuk D. S.; Kovalev I. S.; Zyryanov G. V.; Majee A.; Ranu B. C.; Rusinov V. L.; Chupakhin O. N. Ball-milling: An efficient and green approach for asymmetric organic synthesis. Green Chem. 2020, 22, 302–315. 10.1039/C9GC03414E. - DOI
- Friščić T.; Mottillo C.; Titi H. M. Mechanochemistry for synthesis. Angew. Chem., Int. Ed. 2020, 59, 1018–1029. 10.1002/anie.201906755. - DOI - PubMed
- Porcheddu A.; Colacino E.; De Luca L.; Delogu F. Metal-mediated and metal-catalyzed reactions under mechanochemical conditions. ACS Catal. 2020, 10, 8344–8394. 10.1021/acscatal.0c00142. - DOI
- Howard J. L.; Cao Q.; Browne D. L. Mechanochemistry as an emerging tool for molecular synthesis: what can it offer?. Chem. Sci. 2018, 9, 3080–3094. 10.1039/C7SC05371A. - DOI - PMC - PubMed
- Leonardi M.; Villacampa M.; Menéndez J. C. Multicomponent mechanochemical synthesis. Chem. Sci. 2018, 9, 2042–2064. 10.1039/C7SC05370C. - DOI - PMC - PubMed
-
See also refs (3)–.
-
-
- Gomollón-Bel F. Ten chemical innovations that will change our world. Chem. Int. 2019, 41, 12–17. 10.1515/ci-2019-0203. - DOI
- Krämer K.https://www.chemistryworld.com/news/iupac-names-10-chemistry-innovations....
-
-
For reviews, see:
- Tan D.; Loots L.; Friščić T. Towards medicinal mechanochemistry: evolution of milling from pharmaceutical solid form screening to the synthesis of active pharmaceutical ingredients (APIs). Chem. Commun. 2016, 52, 7760–7781. 10.1039/C6CC02015A. - DOI - PubMed
- Pérez-Venegas M.; Juaristi E. Mechanochemical and mechanoenzymatic synthesis of pharmacologically active compounds: A green perspective. ACS Sustainable Chem. Eng. 2020, 8, 8881–8893. 10.1021/acssuschemeng.0c01645. - DOI
- Ying P.; Yu J.; Su W. Liquid-assisted grinding mechanochemistry in the synthesis of pharmaceuticals. Adv. Synth. Catal. 2021, 363, 1246–1271. 10.1002/adsc.202001245. - DOI
-
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources