YAP drives fate conversion and chemoresistance of small cell lung cancer
- PMID: 34597132
- PMCID: PMC10938532
- DOI: 10.1126/sciadv.abg1850
YAP drives fate conversion and chemoresistance of small cell lung cancer
Abstract
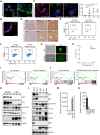
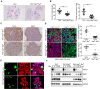
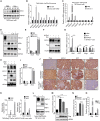
Small cell lung cancer (SCLC) has a high degree of plasticity and is characterized by a remarkable response to chemotherapy followed by the development of resistance. Here, we use a mouse SCLC model to show that intratumoral heterogeneity of SCLC is progressively established during SCLC tumorigenesis. YAP/TAZ and Notch are required for the generation of non-neuroendocrine (Non-NE) SCLC tumor cells, but not for the initiation of SCLC. YAP signals through Notch-dependent and Notch-independent pathways to promote the fate conversion of SCLC from NE to Non-NE tumor cells by inducing Rest expression. In addition, YAP activation enhances the chemoresistance in NE SCLC tumor cells, while the inactivation of YAP in Non-NE SCLC tumor cells switches cell death induced by chemotherapy drugs from apoptosis to pyroptosis. Our study demonstrates that YAP plays critical roles in the establishment of intratumoral heterogeneity and highlights the potential of targeting YAP for chemoresistant SCLC.
Figures






References
-
- Nicholson A. G., Chansky K., Crowley J., Beyruti R., Kubota K., Turrisi A., Eberhardt W. E. E., van Meerbeeck J., Rami-Porta R.; Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions , The international association for the study of lung cancer lung cancer staging project: Proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J. Thorac. Oncol. 11, 300–311 (2016). - PubMed
-
- Gazdar A. F., Bunn P. A., Minna J. D., Small-cell lung cancer: What we know, what we need to know and the path forward. Nat. Rev. Cancer 17, 725–737 (2017). - PubMed
-
- Horn L., Mansfield A. S., Szczęsna A., Havel L., Krzakowski M., Hochmair M. J., Huemer F., Losonczy G., Johnson M. L., Nishio M., Reck M., Mok T., Lam S., Shames D. S., Liu J., Ding B., Lopez-Chavez A., Kabbinavar F., Lin W., Sandler A., Liu S. V.; IMpower133 Study Group , First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 379, 2220–2229 (2018). - PubMed
-
- Ready N. E., Ott P. A., Hellmann M. D., Zugazagoitia J., Hann C. L., de Braud F., Antonia S. J., Ascierto P. A., Moreno V., Atmaca A., Salvagni S., Taylor M., Amin A., Camidge D. R., Horn L., Calvo E., Li A., Lin W. H., Callahan M. K., Spigel D. R., Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: Results from the CheckMate 032 randomized cohort. J. Thorac. Oncol. 15, 426–435 (2020). - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

