Polyphosphate expression by cancer cell extracellular vesicles mediates binding of factor XII and contact activation
- PMID: 34597365
- PMCID: PMC8759128
- DOI: 10.1182/bloodadvances.2021005116
Polyphosphate expression by cancer cell extracellular vesicles mediates binding of factor XII and contact activation
Abstract
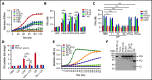
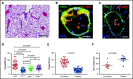
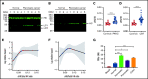
Extracellular vesicles (EV) have been implicated in diverse biological processes, including intracellular communication, transport of nucleic acids, and regulation of vascular function. Levels of EVs are elevated in cancer, and studies suggest that EV may stimulate thrombosis in patients with cancer through expression of tissue factor. However, limited data also implicate EV in the activation of the contact pathway of coagulation through activation of factor XII (FXII) to FXIIa. To better define the ability of EV to initiate contact activation, we compared the ability of EV derived from different cancer cell lines to activate FXII. EV from all cell lines activated FXII, with those derived from pancreatic and lung cancer cell lines demonstrating the most potent activity. Concordant with the activation of FXII, EV induced the cleavage of high molecular weight kininogen (HK) to cleaved kininogen. We also observed that EVs from patients with cancer stimulated FXII activation and HK cleavage. To define the mechanisms of FXII activation by EV, EV were treated with calf intestinal alkaline phosphatase or Escherichia coli exopolyphosphatase to degrade polyphosphate; this treatment blocked binding of FXII to EVs and the ability of EV to mediate FXII activation. In vivo, EV induced pulmonary thrombosis in wild-type mice, with protection conferred by a deficiency in FXII, HK, or prekallikrein. Moreover, pretreatment of EVs with calf intestinal alkaline phosphatase inhibited their prothrombotic effect. These results indicate that polyphosphate mediates the binding of contact factors to EV and that EV-associated polyphosphate may contribute to the prothrombotic effects of EV in cancer.
© 2021 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

