Interactions of Age and Blood Immune Factors and Noninvasive Prediction of Glioma Survival
- PMID: 34597382
- PMCID: PMC8902347
- DOI: 10.1093/jnci/djab195
Interactions of Age and Blood Immune Factors and Noninvasive Prediction of Glioma Survival
Abstract
Background: Tumor-based classification of human glioma portends patient prognosis, but considerable unexplained survival variability remains. Host factors (eg, age) also strongly influence survival times, partly reflecting a compromised immune system. How blood epigenetic measures of immune characteristics and age augment molecular classifications in glioma survival has not been investigated. We assess the prognostic impact of immune cell fractions and epigenetic age in archived blood across glioma molecular subtypes for the first time.
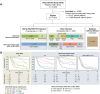
Methods: We evaluated immune cell fractions and epigenetic age in archived blood from the University of California San Francisco Adult Glioma Study, which included a training set of 197 patients with IDH-wild type, 1p19q intact, TERT wild type (IDH/1p19q/TERT-WT) glioma, an evaluation set of 350 patients with other subtypes of glioma, and 454 patients without glioma.
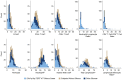
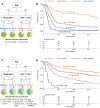
Results: IDH/1p19q/TERT-WT patients had lower lymphocyte fractions (CD4+ T, CD8+ T, natural killer, and B cells) and higher neutrophil fractions than people without glioma. Recursive partitioning analysis delineated 4 statistically significantly different survival groups for patients with IDH/1p19q/TERT-WT based on an interaction between chronological age and 2 blood immune factors, CD4+ T cells, and neutrophils. Median overall survival ranged from 0.76 years (95% confidence interval = 0.55-0.99) for the worst survival group (n = 28) to 9.72 years (95% confidence interval = 6.18 to not available) for the best (n = 33). The recursive partitioning analysis also statistically significantly delineated 4 risk groups in patients with other glioma subtypes.
Conclusions: The delineation of different survival groups in the training and evaluation sets based on an interaction between chronological age and blood immune characteristics suggests that common host immune factors among different glioma types may affect survival. The ability of DNA methylation-based markers of immune status to capture diverse, clinically relevant information may facilitate noninvasive, personalized patient evaluation in the neuro-oncology clinic.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. - PubMed

