Thermally Driven Membrane Phase Transitions Enable Content Reshuffling in Primitive Cells
- PMID: 34597506
- PMCID: PMC8607435
- DOI: 10.1021/jacs.1c06595
Thermally Driven Membrane Phase Transitions Enable Content Reshuffling in Primitive Cells
Abstract
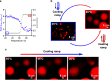
Self-assembling single-chain amphiphiles available in the prebiotic environment likely played a fundamental role in the advent of primitive cell cycles. However, the instability of prebiotic fatty acid-based membranes to temperature and pH seems to suggest that primitive cells could only host prebiotically relevant processes in a narrow range of nonfluctuating environmental conditions. Here we propose that membrane phase transitions, driven by environmental fluctuations, enabled the generation of daughter protocells with reshuffled content. A reversible membrane-to-oil phase transition accounts for the dissolution of fatty acid-based vesicles at high temperatures and the concomitant release of protocellular content. At low temperatures, fatty acid bilayers reassemble and encapsulate reshuffled material in a new cohort of protocells. Notably, we find that our disassembly/reassembly cycle drives the emergence of functional RNA-containing primitive cells from parent nonfunctional compartments. Thus, by exploiting the intrinsic instability of prebiotic fatty acid vesicles, our results point at an environmentally driven tunable prebiotic process, which supports the release and reshuffling of oligonucleotides and membrane components, potentially leading to a new generation of protocells with superior traits. In the absence of protocellular transport machinery, the environmentally driven disassembly/assembly cycle proposed herein would have plausibly supported protocellular content reshuffling transmitted to primitive cell progeny, hinting at a potential mechanism important to initiate Darwinian evolution of early life forms.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

