Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment
- PMID: 34597941
- PMCID: PMC8423808
- DOI: 10.1016/j.esmoop.2021.100274
Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment
Abstract
Background: Cancer patients are at a higher risk of developing severe coronavirus disease 2019 (COVID-19). However, the safety and efficacy of COVID-19 vaccination in cancer patients undergoing treatment remain unclear.
Patients and methods: In this interventional prospective multicohort study, priming and booster doses of the BNT162b2 COVID-19 vaccine were administered 21 days apart to solid tumor patients receiving chemotherapy, immunotherapy, targeted or hormonal therapy, and patients with a hematologic malignancy receiving rituximab or after allogeneic hematopoietic stem cell transplantation. Vaccine safety and efficacy (until 3 months post-booster) were assessed. Anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor-binding domain (RBD) antibody levels were followed over time (until 28 days after the booster) and in vitro SARS-CoV-2 50% neutralization titers (NT50) toward the wild-type Wuhan strain were analyzed 28 days after the booster.
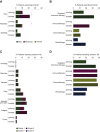
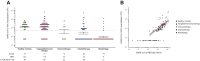
Results: Local and systemic adverse events (AEs) were mostly mild to moderate (only 1%-3% of patients experienced severe AEs). Local, but not systemic, AEs occurred more frequently after the booster dose. Twenty-eight days after the booster vaccination of 197 cancer patients, RBD-binding antibody titers and NT50 were lower in the chemotherapy group {234.05 IU/ml [95% confidence interval (CI) 122.10-448.66] and 24.54 (95% CI 14.50-41.52), respectively} compared with healthy individuals [1844.93 IU/ml (95% CI 1383.57-2460.14) and 122.63 (95% CI 76.85-195.67), respectively], irrespective of timing of vaccination during chemotherapy cycles. Extremely low antibody responses were seen in hematology patients receiving rituximab; only two patients had RBD-binding antibody titers necessary for 50% protection against symptomatic SARS-CoV-2 infection (<200 IU/ml) and only one had NT50 above the limit of detection. During the study period, five cancer patients tested positive for SARS-CoV-2 infection, including a case of severe COVID-19 in a patient receiving rituximab, resulting in a 2-week hospital admission.
Conclusion: The BNT162b2 vaccine is well-tolerated in cancer patients under active treatment. However, the antibody response of immunized cancer patients was delayed and diminished, mainly in patients receiving chemotherapy or rituximab, resulting in breakthrough infections.
Keywords: BNT162b2 COVID-19 vaccination; anti-RBD IgG antibody response; antineoplastic treatment; cancer; safety.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Disclosure MP declares to have an advisory role within Remedus. All other authors have declared no conflicts of interest.
Figures


References
-
- WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ Available at.
-
- Aschwanden C. The false promise of herd immunity for COVID-19. Nature. 2020;587:26–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

