Pharmacology-based ranking of anti-cancer drugs to guide clinical development of cancer immunotherapy combinations
- PMID: 34598713
- PMCID: PMC8485537
- DOI: 10.1186/s13046-021-02111-5
Pharmacology-based ranking of anti-cancer drugs to guide clinical development of cancer immunotherapy combinations
Abstract
The success of antibodies targeting Programmed cell death protein 1 (PD-1) and its ligand L1 (PD-L1) in cancer treatment and the need for improving response rates has led to an increased demand for the development of combination therapies with anti-PD-1/PD-L1 blockers as a backbone. As more and more drugs with translational potential are identified, the number of clinical trials evaluating combinations has increased considerably and the demand to prioritize combinations having potential for success over the ones that are unlikely to be successful is rising. This review aims to address the unmet need to prioritize cancer immunotherapy combinations through comprehensive search of potential drugs and ranking them based on their mechanism of action, clinical efficacy and safety. As lung cancer is one of the most frequently studied cancer types, combinations that showed potential for the treatment of lung cancer were prioritized. A literature search was performed to identify drugs with potential in combination with PD-1/PD-L1 blockers and the drugs were ranked based on their mechanism of action and known clinical efficacy. Nineteen drugs or drug classes were identified from an internal list of lead molecules and were scored for their clinical potential. Efficacy and safety data from pivotal studies was summarized for the selected drugs. Further, overlap of mechanisms of action and adverse events was visualized using a heat map illustration to help screen drugs for combinations. The quantitative scoring methodology provided in this review could serve as a template for preliminary ranking of novel combinations.
Keywords: Cancer; Cancer immunotherapy; Clinical trials; Combination development; Pharmacology.
© 2021. The Author(s).
Conflict of interest statement
VL and CSS are employees of Genentech, Inc and Roche stockholders. AR is an independent consultant who was contracted by Genentech Inc during the course of the review.
Figures
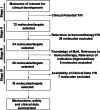
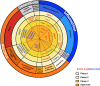


References
-
- Upadhaya S, Neftelino ST, Hodge JP, Oliva C, Campbell JR, Yu JX. Combinations take centre stage in PD1/PDL1 inhibitor clinical trials. Nat Rev Drug Discov. 2021;20:168–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

