Dual checkpoint blockade of CD47 and PD-L1 using an affinity-tuned bispecific antibody maximizes antitumor immunity
- PMID: 34599020
- PMCID: PMC8488710
- DOI: 10.1136/jitc-2021-003464
Dual checkpoint blockade of CD47 and PD-L1 using an affinity-tuned bispecific antibody maximizes antitumor immunity
Abstract
Background: T cell checkpoint immunotherapies have shown promising results in the clinic, but most patients remain non-responsive. CD47-signal regulatory protein alpha (SIRPα) myeloid checkpoint blockade has shown early clinical activity in hematologic malignancies. However, CD47 expression on peripheral blood limits αCD47 antibody selectivity and thus efficacy in solid tumors.
Methods: To improve the antibody selectivity and therapeutic window, we developed a novel affinity-tuned bispecific antibody targeting CD47 and programmed death-ligand 1 (PD-L1) to antagonize both innate and adaptive immune checkpoint pathways. This PD-L1-targeted CD47 bispecific antibody was designed with potent affinity for PD-L1 and moderate affinity for CD47 to achieve preferential binding on tumor and myeloid cells expressing PD-L1 in the tumor microenvironment (TME).
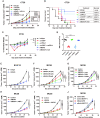
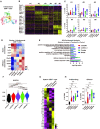
Results: The antibody design reduced binding on red blood cells and enhanced selectivity to the TME, improving the therapeutic window compared with αCD47 and its combination with αPD-L1 in syngeneic tumor models. Mechanistically, both myeloid and T cells were activated and contributed to antitumor activity of αCD47/PD-L1 bispecific antibody. Distinct from αCD47 and αPD-L1 monotherapies or combination therapies, single-cell RNA sequencing (scRNA-seq) and gene expression analysis revealed that the bispecific treatment resulted in unique innate activation, including pattern recognition receptor-mediated induction of type I interferon pathways and antigen presentation in dendritic cells and macrophage populations. Furthermore, treatment increased the Tcf7+ stem-like progenitor CD8 T cell population in the TME and promoted its differentiation to an effector-like state. Consistent with mouse data, the compounds were well tolerated and demonstrated robust myeloid and T cell activation in non-human primates (NHPs). Notably, RNA-seq analysis in NHPs provided evidence that the innate activation was mainly contributed by CD47-SIRPα but not PD-L1-PD-1 blockade from the bispecific antibody.
Conclusion: These findings provide novel mechanistic insights into how myeloid and T cells can be uniquely modulated by the dual innate and adaptive checkpoint antibody and demonstrate its potential in clinical development (NCT04881045) to improve patient outcomes over current PD-(L)1 and CD47-targeted therapies.
Keywords: antibody affinity; immunity; immunotherapy; innate; tumor microenvironment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors are employees of Pfizer which developed antibodies presented in this manuscript.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
