Short tail stories: the hirudin-like factors HLF6 and HLF7 of the Asian medicinal leech, Hirudinaria manillensis
- PMID: 34599360
- PMCID: PMC8516769
- DOI: 10.1007/s00436-021-07316-3
Short tail stories: the hirudin-like factors HLF6 and HLF7 of the Asian medicinal leech, Hirudinaria manillensis
Abstract
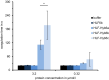
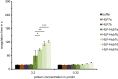
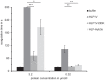
The leech-derived hirudins and hirudin-like factors (HLFs) share a common molecule structure: a short N-terminus, a central globular domain, and an elongated C-terminal tail. All parts are important for function. HLF6 and HLF7 were identified in the Asian medicinal leech, Hirudinaria manillensis. The genes of both factors encode putative splice variants that differ in length and composition of their respective C-terminal tails. In either case, the tails are considerably shorter compared to hirudins. Here we describe the functional analyses of the natural splice variants and of synthetic variants that comprise an altered N-terminus and/or a modified central globular domain. All natural splice variants of HLF6 and HLF7 display no detectable thrombin-inhibitory potency. In contrast, some synthetic variants effectively inhibit thrombin, even with tails as short as six amino acid residues in length. Our data indicate that size and composition of the C-terminal tail of hirudins and HLFs can vary in a great extent, yet the full protein may still retain the ability to inhibit thrombin.
Keywords: Blood coagulation; Hirudin; Hirudin-like factors; Medicinal leeches.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Hirudins of the Asian medicinal leech, Hirudinaria manillensis: same same, but different.Parasitol Res. 2019 Jul;118(7):2223-2233. doi: 10.1007/s00436-019-06365-z. Epub 2019 Jun 11. Parasitol Res. 2019. PMID: 31187225
-
Make it double: identification and characterization of a Tandem-Hirudin from the Asian medicinal leech Hirudinaria manillensis.Parasitol Res. 2022 Oct;121(10):2995-3006. doi: 10.1007/s00436-022-07634-0. Epub 2022 Aug 25. Parasitol Res. 2022. PMID: 36006484 Free PMC article.
-
The hirudin-like factors HLF3 and HLF4-hidden hirudins of European medicinal leeches.Parasitol Res. 2020 Jun;119(6):1767-1775. doi: 10.1007/s00436-020-06697-1. Epub 2020 May 4. Parasitol Res. 2020. PMID: 32363441 Free PMC article.
-
Hirudin as alternative anticoagulant--a historical review.Semin Thromb Hemost. 2002 Oct;28(5):405-14. doi: 10.1055/s-2002-35292. Semin Thromb Hemost. 2002. PMID: 12420235 Review.
-
Thrombin inhibitors of bloodsucking animals.Semin Thromb Hemost. 1996;22(2):203-8. doi: 10.1055/s-2007-999009. Semin Thromb Hemost. 1996. PMID: 8807718 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

