Phase 2 study of axicabtagene ciloleucel in Japanese patients with relapsed or refractory large B-cell lymphoma
- PMID: 34599413
- PMCID: PMC8732921
- DOI: 10.1007/s10147-021-02033-4
Phase 2 study of axicabtagene ciloleucel in Japanese patients with relapsed or refractory large B-cell lymphoma
Abstract
Background: Axicabtagene ciloleucel (axi-cel) is an autologous chimeric antigen receptor T-cell based anti-CD19 therapy. The ZUMA-1 study, multicenter, single-arm, registrational Phase 1/2 study of axi-cel demonstrated high objective response rate in patients with relapsed/refractory large B-cell lymphoma. Here, we present the results of the bridging study to evaluate the efficacy and safety of axi-cel in Japanese patients (JapicCTI-183914).
Methods: This study was the phase 2, multicenter, open-label, single-arm trial. Following leukapheresis, axi-cel manufacturing and lymphodepleting chemotherapy, patients received a single infusion of axi-cel (2.0 × 106 cells/kg). Bridging therapy between leukapheresis and conditioning chemotherapy was not allowed. The primary endpoint was objective response rate.
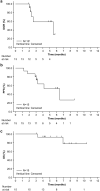
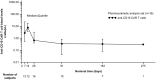
Results: Among 17 enrolled patients, 16 received axi-cel infusion. In the 15 efficacy evaluable patients, objective response rate was 86.7% (95% confidence interval: 59.5-98.3%); complete response/partial response were observed in 4 (26.7%)/9 (60.0%) patients, respectively. No dose-limiting toxicities were observed. Grade ≥ 3 treatment-emergent adverse events occurred in 16 (100%) patients-most commonly neutropenia (81.3%), lymphopenia (81.3%) and thrombocytopenia (62.5%). Cytokine release syndrome occurred in 13 (81.3%) patients (12 cases of grade 1 or 2 and 1 case of grade 4). No neurologic events occurred. Two patients died due to disease progression, but no treatment-related death was observed by the data-cutoff date (October 23, 2019).
Conclusion: The efficacy and safety of axi-cel was confirmed in Japanese patients with relapsed/refractory large B-cell lymphoma who have otherwise limited treatment options.
Trial registration: JapicCTI-183914.
Keywords: Axicabtagene ciloleucel; CD19-specific chimeric antigen receptor; Japan; KTE-C19; Non-Hodgkin lymphoma.
© 2021. The Author(s).
Conflict of interest statement
KK reports honoraria from Kyowa Kirin, Takeda, and MSD, research funds from Celgene, Daiichi Sankyo, Novartis, and Chugai, and scholarship donations from Mundipharma. JK reports research funding from Eisai. KS reports research findings from Kyowa Kirin, Otsuka Pharmaceutical, and Bristol-Myers Squibb, and scholarship donations from Nippon Shinyaku, Zenyaku Kogyo, Chugai, Kyowa Kirin, Takeda, and Eisai. KA reports honoraria from Bristol-Myers Squibb, Celgene, Janssen, and Novartis and research funds from Daiichi Sankyo. KI reports honoraria from Celgene, Novartis, Daiichi Sankyo, and Chugai and research funding from Celgene, Novartis, Daiichi Sankyo, and Chugai. TT reports honoraria from Celgene, Novartis, Bristol Myers Squibb, Janssen, and Takeda, research funds from Daiichi Sankyo, Novartis, Takara Bio, Janssen, and Takeda, and scholarship donations from Takeda and Bristol Myers Squibb. SS and KY are employees of Daiichi-Sankyo and report profit from stock from Daiichi Sankyo. KT reports honoraria from Daiichi Sankyo, Celgene, Takeda, HUYA Bioscience, Zenyaku Kogyo, Yakult, Chugai, Mundipharma, Eisai, and Ono. NU reports honoraria from CMIC, AbbVie, Daiichi Sankyo, Nippon Shinyaku, Celgene, Pfizer, and Astellas. KH reports honoraria from Takeda, Daiichi Sankyo, Towa Pharmaceutical, Meiji Seika Pharma, and Celgene, manuscript fees from Takeda, and scholarship donations from Eisai and Takeda. NF, TS, HS, and YK are employees of Daiichi Sankyo. The other authors have no conflict of interest.
Figures


References
-
- International Agency for Research on Cancer (2020) Japan Source: Globocan 2020. https://gco.iarc.fr/today/data/factsheets/populations/392-japan-fact-she.... Accessed 11 Feb 2021
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

