Characteristics of Growth in Children With Classic Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency During Adrenarche and Beyond
- PMID: 34599587
- PMCID: PMC8764343
- DOI: 10.1210/clinem/dgab701
Characteristics of Growth in Children With Classic Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency During Adrenarche and Beyond
Abstract
Context: Patients with classic congenital adrenal hyperplasia (CAH) often do not achieve their full growth potential. Adrenarche may accelerate bone maturation and thereby result in decreased growth in CAH.
Objective: The study aimed to analyze the impact of growth during adrenarche on final height of adequately treated classic CAH patients.
Methods: This retrospective, multicenter study (4 academic pediatric endocrinology centers) included 41 patients with classical CAH, born 1990-2012. We assessed skeletal maturation (bone age), growth velocity, and (projected) adult height outcomes, and analyzed potential influencing factors, such as sex, genotype, and glucocorticoid therapy.
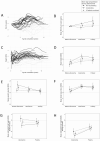
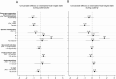
Results: Patients with classic CAH were shorter than peers (-0.4 SDS ± 0.8 SD) and their parents (corrected final height -0.6 SDS ± 1.0 SD). Analysis of growth during adrenarche revealed 2 different growth patterns: patients with accelerating bone age (49%), and patients with nonaccelerating bone age relative to chronological age (BA-CA). Patients with accelerating BA-CA were taller than the normal population during adrenarche years (P = 0.001) and were predicted to achieve lower adult height SDS (-0.9 SDS [95% CI, -1.3; -0.5]) than nonaccelerating patients when assessed during adrenarche (0.2 SDS [95% CI, -0.3; 0.8]). Final adult height was similarly reduced in both accelerating and nonaccelerating BA-CA groups (-0.4 SDS [95% CI, -0.9; 0.1] vs -0.3 SDS [95% CI, [-0.8; 0.1]).
Conclusion: Patients with and without significant bone age advancement, and thus differing height prediction during adrenarche, showed similar (predicted) final height when reassessed during pubertal years. Bone age alone should not be used during adrenarche as clinical marker for metabolic control in CAH treatment.
Keywords: adrenarche; classic CAH; final height prediction; growth.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
-
- Hargitai G, Sólyom J, Battelino T, et al. ; MEWPE-CAH Study Group . Growth patterns and final height in congenital adrenal hyperplasia due to classical 21-hydroxylase deficiency. Results of a multicenter study. Horm Res. 2001;55(4):161-171. - PubMed
-
- Allen DB. Growth suppression by glucocorticoid therapy. Endocrinol Metab Clin North Am. 1996;25(3):699-717. - PubMed
-
- Bonfig W, Bechtold S, Schmidt H, Knorr D, Schwarz HP. Reduced final height outcome in congenital adrenal hyperplasia under prednisone treatment: deceleration of growth velocity during puberty. J Clin Endocrinol Metab. 2007;92(5):1635-1639. - PubMed
-
- Hindmarsh PC. Management of the child with congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23(2):193-208. - PubMed

