Magnetofluidic immuno-PCR for point-of-care COVID-19 serological testing
- PMID: 34600203
- PMCID: PMC8458161
- DOI: 10.1016/j.bios.2021.113656
Magnetofluidic immuno-PCR for point-of-care COVID-19 serological testing
Abstract
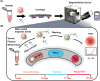
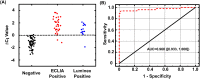
Serological tests play an important role in the fight against Coronavirus Disease 2019 (COVID-19), including monitoring the dynamic immune response after vaccination, identifying past infection and determining community infection rate. Conventional methods for serological testing, such as enzyme-linked immunosorbent assays and chemiluminescence immunoassays, provide reliable and sensitive antibody detection but require sophisticated laboratory infrastructure and/or lengthy assay time. Conversely, lateral flow immunoassays are suitable for rapid point-of-care tests but have limited sensitivity. Here, we describe the development of a rapid and sensitive magnetofluidic immuno-PCR platform that can address the current gap in point-of-care serological testing for COVID-19. Our magnetofluidic immuno-PCR platform automates a magnetic bead-based, single-binding, and one-wash immuno-PCR assay in a palm-sized magnetofluidic device and delivers results in ∼30 min. In the device, a programmable magnetic arm attracts and transports magnetically-captured antibodies through assay reagents pre-loaded in a companion plastic cartridge, and a miniaturized thermocycler and a fluorescence detector perform immuno-PCR to detect the antibodies. We evaluated our magnetofluidic immuno-PCR with 108 clinical serum/plasma samples and achieved 93.8% (45/48) sensitivity and 98.3% (59/60) specificity, demonstrating its potential as a rapid and sensitive point-of-care serological test for COVID-19.
Keywords: COVID-19; Immuno-PCR; Magnetofluidic device; Point-of-care; Serological testing.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., Jiang K., Arunkumar G.A., Jurczyszak D., Polanco J., Bermudez-Gonzalez M., Kleiner G., Aydillo T., Miorin L., Fierer D.S., Lugo L.A., Kojic E.M., Stoever J., Liu S.T.H., Cunningham-Rundles C., Felgner P.L., Moran T., García-Sastre A., Caplivski D., Cheng A.C., Kedzierska K., Vapalahti O., Hepojoki J.M., Simon V., Krammer F. Nat. Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. - DOI - PMC - PubMed
-
- Chen F.E., Trick A.Y., Hsieh W., Shin D.J., Chen L., Chang E., Kaushik A., Wang T.H. Int. Conf. Solid-State Sensors, Actuators Microsystems Eurosensors XXXIII. 2019. TRANSDUCERS 2019 EUROSENSORS XXXIII 1116–1119, 2019 20th. - DOI
-
- CSSE COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins Univeristy (JHU) https://coronavirus.jhu.edu/map.html URL. 12.30.20.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

