The Nrf2-NLRP3-caspase-1 axis mediates the neuroprotective effects of Celastrol in Parkinson's disease
- PMID: 34600334
- PMCID: PMC8487081
- DOI: 10.1016/j.redox.2021.102134
The Nrf2-NLRP3-caspase-1 axis mediates the neuroprotective effects of Celastrol in Parkinson's disease
Abstract
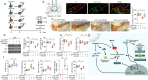
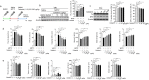
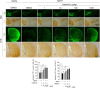
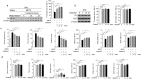
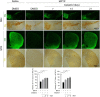
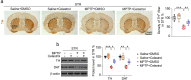
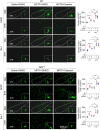
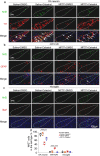
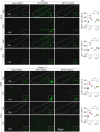
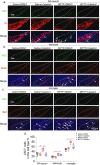
Parkinson's disease (PD) is a chronic neurodegenerative disorder that is characterized by motor symptoms as a result of a loss of dopaminergic neurons in the substantia nigra pars compacta (SNc), accompanied by chronic neuroinflammation, oxidative stress, formation of α-synuclein aggregates. Celastrol, a potent anti-inflammatory and anti-oxidative pentacyclic triterpene, has emerged as a neuroprotective agent. However, the mechanisms by which celastrol is neuroprotective in PD remain elusive. Here we show that celastrol protects against dopamine neuron loss, mitigates neuroinflammation, and relieves motor deficits in MPTP-induced PD mouse model and AAV-mediated human α-synuclein overexpression PD model. Whole-genome deep sequencing analysis revealed that Nrf2, NLRP3 and caspase-1 in SNc may be associated with the neuroprotective actions of celastrol in PD. By using multiple genetically modified mice (Nrf2-KO, NLRP3-KO and Caspase-1-KO), we identified that celastrol inhibits NLRP3 inflammasome activation, relieves motor deficits and nigrostriatal dopaminergic degeneration through Nrf2-NLRP3-caspase-1 pathway. Taken together, these findings suggest that Nrf2-NLRP3-caspase-1 axis may serve as a key target of celastrol in PD treatment, and highlight the favorable properties of celastrol for neuroprotection, making celastrol as a promising disease-modifying agent for PD.
Keywords: Caspase-1; Celastrol; NLRP3; Nrf2; Parkinson's disease; α-synuclein.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





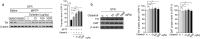
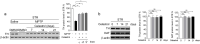







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

