Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study
- PMID: 34600602
- PMCID: PMC9377412
- DOI: 10.1016/S1470-2045(21)00402-2
Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study
Abstract
Background: The majority of patients with metastatic castration-resistant prostate cancer (mCRPC) will have disease progression of a uniformly fatal disease. mCRPC is driven by both activated androgen receptors and elevated intratumoural androgens; however, the current standard of care is therapy that targets a single androgen signalling mechanism. We aimed to investigate the combination treatment using apalutamide plus abiraterone acetate, each of which suppresses the androgen signalling axis in a different way, versus standard care in mCRPC.
Methods: ACIS was a randomised, placebo-controlled, double-blind, phase 3 study done at 167 hospitals in 17 countries in the USA, Canada, Mexico, Europe, the Asia-Pacific region, Africa, and South America. We included chemotherapy-naive men (aged ≥18 years) with mCRPC who had not been previously treated with androgen biosynthesis signalling inhibitors and were receiving ongoing androgen deprivation therapy, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and a Brief Pain Inventory-Short Form question 3 (ie, worst pain in the past 24 h) score of 3 or lower. Patients were randomly assigned (1:1) via a centralised interactive web response system with a permuted block randomisation scheme (block size 4) to oral apalutamide 240 mg once daily plus oral abiraterone acetate 1000 mg once daily and oral prednisone 5 mg twice daily (apalutamide plus abiraterone-prednisone group) or placebo plus abiraterone acetate and prednisone (abiraterone-prednisone group), in 28-day treatment cycles. Randomisation was stratified by presence or absence of visceral metastases, ECOG performance status, and geographical region. Patients, the investigators, study team, and the sponsor were masked to group assignments. An independent data-monitoring committee continually monitored data to ensure ongoing patient safety, and reviewed efficacy data. The primary endpoint was radiographic progression-free survival assessed in the intention-to-treat population. Safety was reported for all patients who received at least one dose of study drug. This study is completed and no longer recruiting and is registered with ClinicalTrials.gov, number NCT02257736.
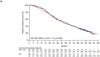
Findings: 982 men were enrolled and randomly assigned from Dec 10, 2014 to Aug 30, 2016 (492 to apalutamide plus abiraterone-prednisone; 490 to abiraterone-prednisone). At the primary analysis (median follow-up 25·7 months [IQR 23·0-28·9]), median radiographic progression-free survival was 22·6 months (95% CI 19·4-27·4) in the apalutamide plus abiraterone-prednisone group versus 16·6 months (13·9-19·3) in the abiraterone-prednisone group (hazard ratio [HR] 0·69, 95% CI 0·58-0·83; p<0·0001). At the updated analysis (final analysis for overall survival; median follow-up 54·8 months [IQR 51·5-58·4]), median radiographic progression-free survival was 24·0 months (95% CI 19·7-27·5) versus 16·6 months (13·9-19·3; HR 0·70, 95% CI 0·60-0·83; p<0·0001). The most common grade 3-4 treatment-emergent adverse event was hypertension (82 [17%] of 490 patients receiving apalutamide plus abiraterone-prednisone and 49 [10%] of 489 receiving abiraterone-prednisone). Serious treatment-emergent adverse events occurred in 195 (40%) patients receiving apalutamide plus abiraterone-prednisone and 181 (37%) patients receiving abiraterone-prednisone. Drug-related treatment-emergent adverse events with fatal outcomes occurred in three (1%) patients in the apalutamide plus abiraterone-prednisone group (2 pulmonary embolism, 1 cardiac failure) and five (1%) patients in the abiraterone-prednisone group (1 cardiac failure and 1 cardiac arrest, 1 mesenteric arterial occlusion, 1 seizure, and 1 sudden death).
Interpretation: Despite the use of an active and established therapy as the comparator, apalutamide plus abiraterone-prednisone improved radiographic progression-free survival. Additional studies to identify subgroups of patients who might benefit the most from combination therapy are needed to further refine the treatment of mCRPC.
Funding: Janssen Research & Development.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests FS received financial support from Janssen for the ACIS study; he reports grants or contracts, consulting fees, and payment or honoraria for lectures, presentations, speakers bureaus, and manuscript writing or educational events from Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Janssen, Myovant, Novartis, and Sanofi, outside of the submitted work. EE received financial support from Janssen for the ACIS study; she reports grants or contracts from Astellas, AstraZeneca, Janssen, Merck, Oric, and Sanofi; consulting fees from Astellas, AstraZeneca, Bayer, Janssen, Merck, Pfizer, and Sanofi; payment or honoraria for lectures, presentations, speakers bureaus, and manuscript writing or educational events from Bayer, Janssen, and Sanofi; financial support for attending meetings, travel, or both, from Astellas, Janssen, and Sanofi; participated in Data Safety Monitoring Board or Advisory Board meetings for AstraZeneca, Janssen, Merck, Pfizer, and Sanofi; and reports leadership or fiduciary roles in other board, society, committee, or advocacy group, paid or unpaid, for the European Society of Medical Oncology, outside of the submitted work. GA received financial support from Janssen for the ACIS study; he reports grants or contracts from Astellas and Janssen; consulting fees from Astellas, Bayer, Beigene, Clovis, Janssen, Novartis, Pfizer, and Sanofi; payment or honoraria for lectures, presentations, speakers bureaus, and manuscript writing or educational events from Astellas, Bayer, Janssen, Novartis, and Sanofi; financial support for attending meetings, travel, or both, from Astellas, Bayer, Janssen, Novartis, Pfizer, and Sanofi; participated in Data Safety Monitoring Board or Advisory Board meetings for Astellas, Bayer, Janssen, Novartis, Pfizer, and Sanofi; received royalties from Janssen paid to the Institute of Cancer Research; and is included on the list of rewards to discoverers of abiraterone, outside of the submitted work. TWF received grants or contracts from Agensys, Aragon Pharmaceuticals, Astellas Pharma, AstraZeneca–MedImmune, Bristol Myers Squibb, Bavarian Nordic, Dendreon, Exelixis, GTx, Janssen Oncology, La Roche-Posay, Lilly, Medivation, Merck, Novartis, Pfizer, Roche–Genentech, Sanofi, Sotio, Seagen, Seattle Genetics, and Tokai Pharmaceuticals; consulting fees from Aurora Oncology, Janssen Oncology, and Seattle Genetics; filed two patents (via the University of Colorado) related to early-stage bladder cancer treatment and detection for which he is an inventor, neither of which are currently commercialised or in clinical trials; and is the founder and a stockholder for Aurora Oncology, all outside of the submitted work. OBG received consulting fees from Bayer and Janssen and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, and educational events from Janssen, all outside of the submitted work. SO reports consulting fees, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, and support for attending meetings, travel, or both from Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck, Novartis, Pfizer, and Sanofi and participated in Data Safety Monitoring Board or Advisory Board meetings for Astellas, Janssen, Roche, and Sanofi, all outside of the submitted work. TS received consulting fees and payment or honoraria for lectures, presentations, speakers bureaus, and manuscript writing or educational events from Astellas Oncology, Bayer Health, Janssen, and Sanofi, all outside of the submitted work. HS received financial support from Janssen for the ACIS study; he reports grants or contracts from Asahi Kasei, Bayer, Daiichi Sankyo, Kissei, Nippon Kayaku, Nippon Shinyaku, Ono, Sanofi, Taiho, and Takeda; consulting fees from Astellas, AstraZeneca, Bayer, Eli Lilly, Janssen, MSD, Nihon Medi-Physics, Roche–Chugai, and Sanofi; payment or honoraria for lectures, presentations, speakers bureaus, and manuscript writing or educational events from Astellas, AstraZeneca, Bayer, Janssen, Merck Biopharma, MSD, Nippon Shinyaku, Ono, Otsuka, Pfizer, Sanofi, and Takeda; support for attending meetings, travel, or both from Astellas, Bayer, Eli Lilly, Janssen, and Sanofi; and participated in Advisory Board meetings for Astellas, Bayer, Janssen, Roche–Chugai, and Sanofi, outside of the submitted work. DER received support from Janssen for the ACIS study; she reports uncompensated participation in advisory boards for AstraZeneca, Bayer, Genentech, Janssen, and Myovant, outside of the submitted work. DW, KY, PDP, SB-M, SL, JL, ST, KBB, SDM, and SAM are employed by Janssen Research & Development, and hold stock in Johnson & Johnson. FF declares no competing interests.
Figures



Comment in
-
Drug development in metastatic prostate cancer: lessons from ACIS.Lancet Oncol. 2021 Nov;22(11):1487-1488. doi: 10.1016/S1470-2045(21)00458-7. Epub 2021 Sep 30. Lancet Oncol. 2021. PMID: 34600603 No abstract available.
-
Radiographic progression-free survival in the ACIS trial for prostate cancer.Lancet Oncol. 2022 Jan;23(1):e3. doi: 10.1016/S1470-2045(21)00710-5. Lancet Oncol. 2022. PMID: 34973229 No abstract available.
-
Radiographic progression-free survival in the ACIS trial for prostate cancer.Lancet Oncol. 2022 Jan;23(1):e4. doi: 10.1016/S1470-2045(21)00719-1. Lancet Oncol. 2022. PMID: 34973231 No abstract available.
-
Radiographic progression-free survival in the ACIS trial for prostate cancer - Authors' reply.Lancet Oncol. 2022 Jan;23(1):e5-e6. doi: 10.1016/S1470-2045(21)00723-3. Lancet Oncol. 2022. PMID: 34973233 No abstract available.
References
-
- James ND, Spears MR, Clarke NW, et al. Survival with newly diagnosed metastatic prostate cancer in the "docetaxel era": data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur Urol 2015; 67: 1028–38. - PubMed
-
- Yamaoka M, Hara T, Kusaka M. Overcoming persistent dependency on androgen signaling after progression to castration-resistant prostate cancer. Clin Cancer Res 2010; 16: 4319–24. - PubMed
-
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015; 16: 152–60. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

